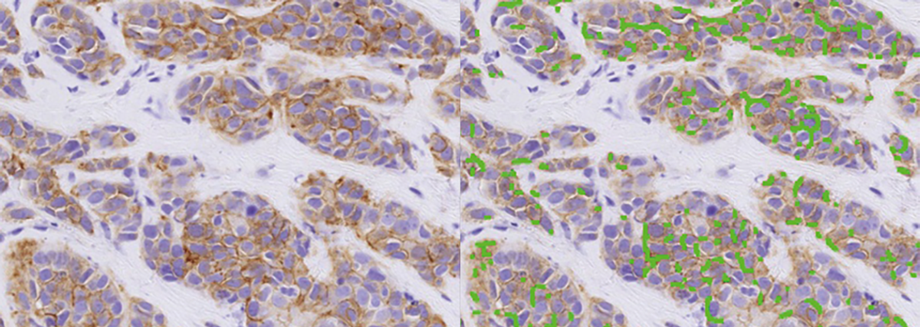
Visiopharm today announces that it will be entering into a research pilot with Massachusetts General Hospital (MGH) to investigate the use of AI-powered precision pathology solutions for quality assurance of immunohistochemistry (IHC) interpretations.
This research agreement will first focus on the utilization of Visiopharm’s AI-driven HER2 algorithm to review specimens in-tandem with traditional IHC and FISH testing. The algorithm has shown the potential to identify tumors with a so-called HER2-low status, a traditionally challenging group to identify through manual IHC interpretation.
A secondary phase of the agreement will aim to expand the research capabilities to other analysis algorithms, or APPs, in the Oncotopix software, which include additional breast cancer biomarkers like ER, PR, Ki67 and H&E metastasis. These APPs are already IVDR certified in Europe, where they are used in a clinical setting.
Martin Kristensson, SVP Clinical Strategy, Visiopharm said:
“The proper classification of HER2 status in stained tissue sections is critical to precision pathology and personalized medicine of cancer patients. Borderline cases require additional timely and costly assessments, including reflex, in situ hybridization (ISH) testing. This collaboration is another exciting milestone for Visiopharm, expanding our research work with leading institutions throughout the United States and Europe. Our AI-powered software solutions and APPs will support the robust needs and requirements of MGH throughout this pilot.”
Amanda Lowe, Managing Director, Americas, Visiopharm, said:
“This is another powerful example of research being applied to translate the value of AI-powered digital pathology; a critical need in driving greater value, efficiency, and precision into pathology. Dr. Lennerz has great passion and conviction to moving the digital pathology industry forward and we are proud to be collaborating with him and the team and MGH.
Dr. Jochen K. Lennerz, Medical Director, Center for Integrated Diagnostics, Massachusetts General Hospital:
“Precise interpretation is crucial – especially for identification of patients with HER2-low expressing tumors. These patients form an important subgroup that benefit from novel treatment strategies. The identification of this population has been traditionally challenging and in this research project, we aim to understand what computational tools could help improve identification of these patients.”
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs provide decision support. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 750 customer accounts and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, The Netherlands and United States.
For enquiries:
Johanne Louise Brændgaard
Chief Marketing Officer
jlb@visiopharm.com
 Visiopharm
Categories: Press Releases
18678
Deep Bio and Visiopharm announce a collaborative integration of a clinical-grade AI prostate cancer solution with a leading digital pathology platform
Visiopharm
Categories: Press Releases
18678
Deep Bio and Visiopharm announce a collaborative integration of a clinical-grade AI prostate cancer solution with a leading digital pathology platform
Deep Bio, a leading AI biotech dedicated to cancer diagnosis, and Visiopharm, a world leader in AI-powered image analysis and tissue mining for research and diagnostics, announced a strategic partnership to provide pathologists access to the latest AI-powered prostate cancer solution.
AI-powered cancer diagnostics has been proving its utility not only in research, but increasingly in supporting and improving pathologists’ decisions, workflow efficiency, and diagnostic precision. Deep Bio’s deep learning-based CE-IVDD prostate cancer diagnosis support software, DeepDx® Prostate, empowers pathologists by identifying cancerous areas and grading their severity providing a Gleason scoring.
With this collaboration, DeepDx® strengthens Visiopharm’s diagnostic offering to its European diagnostic customers. This application expands pathologists’ accessibility to the latest cutting-edge H&E based AI solutions for prostate cancer, which today is a large diagnostic indication that is both time– and labour intensive in diagnostic pathology labs. With its extensive AI-based image analysis solutions for multiple cancer types, Visiopharm’s platform is contributing to the global adoption of digital pathology and AI.
The first phase of integration of DeepDx® into Visiopharm’s digital pathology platform has been completed. In the next phases, Visiopharm will together with Deep Bio scale up the solution as interest grows. The two companies plan to add additional AI cancer pathology solutions over time for prostate and breast, among others.
The second phase of integration will aim for tighter interoperability among the platform and AI solutions, helping both companies to better promote and distribute to healthcare institutions around the world.
Sun Woo Kim, CEO of Deep Bio, said:
“Digital transformation in healthcare is not a new concept anymore. Pathology, which has been slow to undergo digital transformation, is fast becoming digitalized due to a diverse range of AI solutions now available for implementation and validation. Our dedication to AI for digital pathology has taken a major step forward with this collaboration and we are pleased to offer our latest technology to more labs and hospitals across the world through this opportunity. We will continue to support medical professionals to optimize their decisions which ultimately lead to the best patient care.”
Michael Grunkin, CEO of Visiopharm, said:
“We are seeing a lot of demand for this particular application among both our clinical research- and diagnostic customers and partners. In diagnostic workflows, this APP has the potential to support pathologists in automating time-consuming and repetitive work, while improving turnaround time and standardization. This partnership with Deep Bio is a good example of how our scanner, LIMS, and PACS agnostic platform allow our users to benefit both from our own apps and best-in-class apps from our growing partner network, to gain access to a full diagnostic menu.”
About Deep Bio
Deep Bio Inc. is an AI healthcare company with in-house expertise in deep learning and cancer pathology. Our vision is to radically improve efficiency and accuracy of pathologic cancer diagnosis and prognosis, by equipping pathologists with deep learning-based IVD SaMDs1 (In Vitro Diagnostics Software as a Medical Device), for optimal cancer treatment decisions. Deep Bio is also actively engaged in the research space and maintains ongoing collaborations with top US medical centers. To learn more, visit www.deepbio.co.kr.
DeepDx® Prostate is a clinically-validated AI for prostate core needle biopsy tissue image analysis. Whole-slide images (WSIs) of H&E-stained biopsy tissue specimens are analyzed for prostate cancer, Gleason scores and grade groups. Extensively tested at 4 US CLIA labs (> 700k cores between 2019 and 2021), DeepDx® Prostate can alleviate the shortage of pathologists and the resultant increase in workload, while reducing diagnostic subjectivity and variability
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs provide decision support. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 750 customer accounts and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, The Netherlands and United States.
For enquiries:
Johanne Louise Brændgaard
Chief Marketing Officer
jlb@visiopharm.com
 Visiopharm
Categories: Press Releases
18634
Standard BioTools and Visiopharm announce partnership for the development of complete image analysis solutions to be packaged with Hyperion and Hyperion+ Imaging Systems
Visiopharm
Categories: Press Releases
18634
Standard BioTools and Visiopharm announce partnership for the development of complete image analysis solutions to be packaged with Hyperion and Hyperion+ Imaging Systems
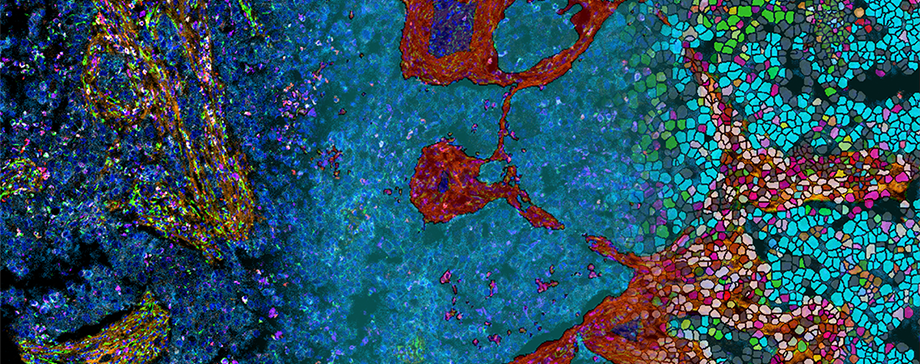
Standard BioTools Inc. (Nasdaq:LAB), driven by a bold purpose – unleashing tools to accelerate breakthroughs in human health – and Visiopharm today announced a collaboration to pair the company’s Hyperion™ and Hyperion+™ Imaging Systems with Visiopharm Phenoplex™ analysis software for simplified image processing and enhanced spatial pathology insights, building on the success of a co-marketing agreement announced last year.
Visiopharm, a leader in AI-driven digital pathology analysis software, and Standard BioTools, a life science preferred provider of innovative tools for exploratory and translational research, have worked together to develop the first of several specific algorithms and workflows that will provide out-of-the-box solutions and accelerate delivery of results for high-plex imaging studies.
The unique Standard BioTools™ single-step staining process and simultaneous 40-plex imaging deliver biomarker-rich data in a single scan for tissue microarrays and formalin-fixed, paraffin-embedded tissue sections, providing enough datapoints to thoroughly phenotype and decipher cellular composition and function. Integrating Phenoplex into Hyperion and Hyperion+ Imaging Systems will bring a powerful, unified image analysis pipeline to Imaging Mass Cytometry™ (IMC™) users, who will be able to more easily extract and more quickly act on meaningful biological data from their IMC investigations.
This close partnership offers one of the most effective solutions to date, in which Visiopharm software reads native MCD files, the output image file from Standard BioTools imaging systems, directly linking MCD™ Viewer to Phenoplex software. Phenoplex then employs the Visiopharm file conversion tool to create pyramidal TIFF images out of MCD files for large image formats that contain more markers for deeper analysis.
The Phenoplex workflow includes an AI tissue segmentation step, an AI-based nuclear segmentation step, cellular boundary detection that can include use of the Standard BioTools IMC Cell Segmentation Kit, cellular phenotyping, data exploration and image QC, spatial analyses, and a wide range of data/image export formats.
The powerful combination of Standard BioTools proven high-plex imaging with successful AI-driven software from Visiopharm will provide a simple and efficient way for translational and clinical researchers to generate and analyze high-plex spatial data from the tumor microenvironment, making it easier to investigate therapeutic and drug responses and arrive at confident decisions impacting healthcare.
Louise Armstrong, Chief Commercial Officer, Visiopharm, said:
“We are delighted to embark on this partnership with Standard BioTools to bring our Phenoplex image analysis to Hyperion Imaging System users everywhere. We have been working to improve and simplify the image analysis of high-plex images like IMC imagery and look forward to collaborating on evolving our analysis solution to meet the needs of researchers in this space.”
Michael Egholm, President and Chief Executive Officer, Standard BioTools, said:
“With this collaboration, Standard BioTools becomes one of the first companies to bundle a system with a complete solution. This seamless pipeline transition from instrument to data analysis will be a big step for multiplex imaging, leading the way for high-plex adoption so customers no longer need to spend their time piecing together freeware to develop a pipeline.”
Use of forward-looking statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, among others, statements regarding the potential benefits of research conducted using Standard BioTools products and technologies and anticipated benefits to Standard BioTools of an expanded collaboration. Forward-looking statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from currently anticipated results, including but not limited to risks relating to interruptions or delays in the supply of components or materials for, or manufacturing of, Standard BioTools products; potential product performance and quality issues; intellectual property risks; competition; uncertainties in contractual relationships; and reductions in research and development spending or changes in budget priorities by customers. Information on these and additional risks and uncertainties and other information affecting Standard BioTools’ business and operating results is contained in its Annual Report on Form 10-K for the year ended December 31, 2021, and in its other filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof. Standard BioTools disclaims any obligation to update these forward-looking statements except as may be required by law.
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs provide decision support. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 750 customer accounts and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, The Netherlands and United States.
For enquiries:
Johanne Louise Brændgaard
Chief Marketing Officer
jlb@visiopharm.com
About Standard BioTools
Standard BioTools Inc. (Nasdaq:LAB), previously known as Fluidigm Corporation, is driven by a bold purpose – unleashing tools to accelerate breakthroughs in human health. Standard BioTools has an established portfolio of essential, standardized next-generation technologies that help biomedical researchers develop medicines faster and better. As a leading solutions provider, the company provides reliable and repeatable insights in health and disease using its proprietary mass cytometry and microfluidics technologies, which help transform scientific discoveries into better patient outcomes. Standard BioTools works with leading academic, government, pharmaceutical, biotechnology, plant and animal research, and clinical laboratories worldwide, focusing on the most pressing needs in translational and clinical research, including oncology, immunology, and immunotherapy. Learn more at www.standardbio.com or connect with us on Twitter®, Facebook®, LinkedIn, and YouTube™. Standard BioTools, the Standard BioTools logo, Fluidigm, the Fluidigm logo, “Unleashing tools to accelerate breakthroughs in human health,” Hyperion, Hyperion+, Imaging Mass Cytometry, IMC, and MCD are trademarks and/or registered trademarks of Standard BioTools Inc. or its affiliates in the United States and/or other countries. All other trademarks are the sole property of their respective owners. Standard BioTools products are provided for Research Use Only. Not for use in diagnostic procedures.
 Visiopharm
Categories: Press Releases
18617
Visiopharm introduces Phenoplex™, a unified solution for a better multiplex image analysis workflow
Visiopharm
Categories: Press Releases
18617
Visiopharm introduces Phenoplex™, a unified solution for a better multiplex image analysis workflow
Visiopharm, a world leader in AI-driven digital pathology image analysis software, today introduced Phenoplex ™, their latest innovation as part of their Oncotopix® Discovery platform.
Phenoplex offers a novel easy-to-use unified workflow for multiplex tissue image analysis. Designed to meet the needs of scientists, it simplifies the analysis pipeline from multiple software suites into one workflow and enables users to continuously review their data to get to meaningful results.
Phenoplex is a complete workflow with built-in tools to:
- Analyse multiplex and highplex images
- Import files to rapidly visualize and QC biomarkers and biomarker groups
- Verify and quantify cellular phenotypes in each tissue region
- Interactively plot connecting multiplex images with graphs/plots and a cell gallery
- Produce and export the data, plots, and imagery needed for manuscript publication
As part of the Oncotopix Discovery AI-based research platform, Phenoplex supports the same flexibility to design the required analysis pipelines without requiring coding skills. Phenoplex analysis works with all major multiplex image file formats, whole-slide images and large datasets.
“Phenoplex embodies Visiopharm’s commitment to multiplex analysis and support for the immuno-oncology community,” said Louise Armstrong, Chief Commercial Officer at Visiopharm. “This new workflow allows us to continue to refine, improve, and create tools within each workflow stage. What researchers need is a unified workflow in one software package that can address all the steps required to deliver meaningful biological results that are needed. Phenoplex will be a trusted companion for anyone needing meaningful data from these complex images.”
Phenoplex was developed to support the critical field of cancer immunology. The growth of immunotherapy as the fifth pillar of cancer treatment has driven a need to better understand the role of the immune system in solid tumor therapies. Understanding the spatial biology of tumors has become crucial for researchers and the main method is the use of multi-marker staining and multiplexed imaging methods in FFPE sections to perform multiplex phenotyping of the cells in situ as orthogonal work done by flow cytometry of suspension samples.
In recent years there have been a number of advanced technologies introduced to perform highplex (10 to 40 or more markers in one section) staining and imaging of tissue sections, preserving the spatial features of cell populations. However, as the ‘plex level of imaging systems has grown, so too has the complexity of the image analysis required to make sense of all this data. Phenoplex was developed, in part, to address the needs of the immuno-oncology field and enable a simpler way to extract important biological information from these complex multiplex images.
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs provide decision support. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 750 customer accounts and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, The Netherlands and United States.
For enquiries:
Johanne Louise Brændgaard
Chief Marketing Officer
jlb@visiopharm.com
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
