The importance of hot-spots
As discussed in the recent post on managing tumor heterogeneity, it is widely considered best practice to determine the Ki-67 proliferative index in a hot-spot of biomarker expression. A growing body of scientific evidence support that correct identification of hot-spots is essential to achieve sufficient interpretive accuracy for predictive and/or prognostic use [1,2]. Hot-spot scoring has recently been adopted in both Swedish and Danish clinical guidelines for breast cancer [3,4].
Heterogeneity is a general challenge
Heterogeneity with respect to expression levels is evident for many tissue biomarkers. Although it may be less obvious how to technically determine heterogeneity and hot-spots for membrane markers, or markers that express in several sub-cellular compartments, it is likely that the ability to visualize and quantify heterogeneity and identify hot-spots will be generally important to cancer research, drug development, biomarker validation, and in diagnostics. To further explore this, we developed a general research tool for quantification of tissue biomarkers, including generation of heatmaps of expression and identification of hot-spots..
The definition of a hot-spot
As an example, this general research tool was used to create an APP for visualizing heatmaps for Ki67 expression across entire tissue sections, and determine the location of hot-spots. Although this seems an intuitively appealing approach for managing heterogeneity, it generates new questions relating to the definition of a hot-spot. Clinical guidelines are usually defining a hot-spot as a square of fixed dimensions, or recommend counting a certain number of cells around the hot-spot. For their presentation at the 29th European Congress of Pathology, Omanovic and Schönauwere comparing five different approaches to defining a hot-spot as outlined in the figure below. Note how the hot spot location and area changes between methods, and that heatmap area and count can be defined along the iso-curve of the biomarker response.
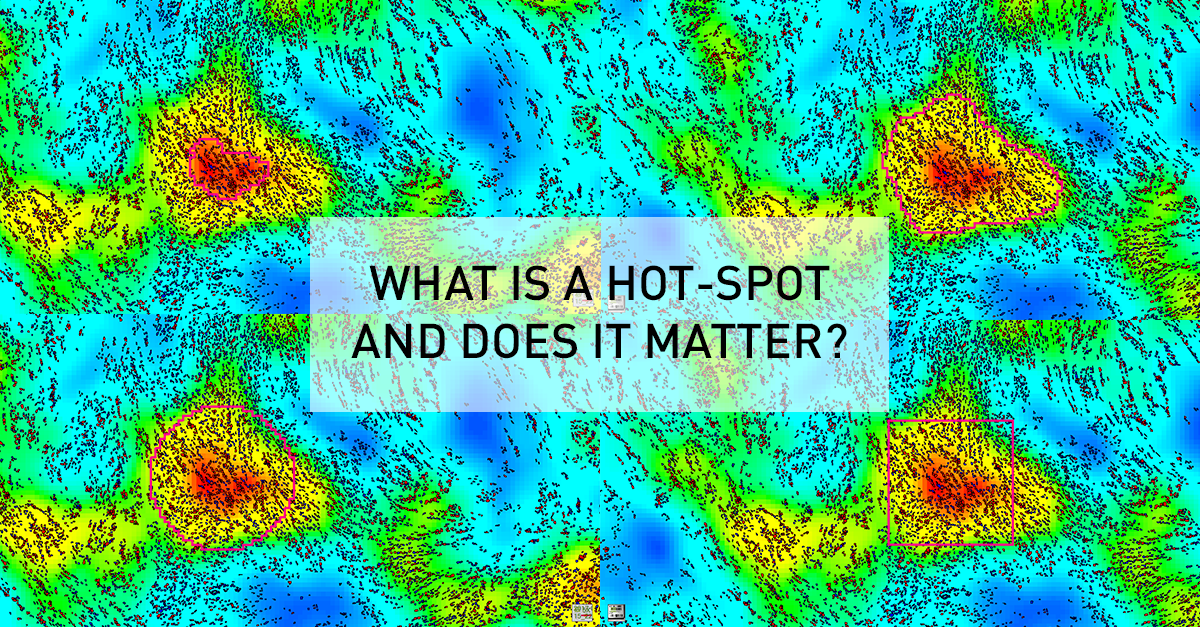
Need for a standardized approach
In the study above, they found that different definitions as well as area of hot-spots has a statistically significant impact on the proliferation index. Therefore, the fact that different guidelines and hot-spot definitions are adopted in clinical practice and for research purposes, also means that results cannot be compared. Thus, it will difficult to define generally applicable clinical standards (e.g. cut-offs) until there is a consensus on a unified / optimal definition of a hot-spot.
Clinical relevance
Apart from the need for standardization, there is also a need to understand to the impact of the observed differences on the predictive or prognostic power of tissue-based assays. At Karolinska University Hospital, Johan Hartman et. al. are currently exploring this in a study design with a longitudinal cohort, clinical outcomes and access to sequencing data. The outcome of this study will hopefully provide insights allowing for a recommendation of an optimal definition of hot-spots, for understanding the impact on diagnostic/prognostic accuracy, and to what extent a modern tissue-diagnostic approach provide statistically independent information compared to molecular methods.
References:
3.KVAST, Swedish breast cancer guidelines, 2018 (in Swedish)
4.DBCG – Danish Guidelines, May 2017 (in Danish).
Michael Grunkin Categories: Blog 5959 Managing Tumor Heterogeneity for Quantification of Biomarker ResponseTumor heterogeneity in research and diagnostics
Diagnostic pathologists and scientists often rely on tissue data for important diagnostic-, research-, or even business- decisions. One of the major concerns relate to tumor heterogeneity in the context of quantifying tissue biomarker response. Questions revolve around how to reliably visualize, quantify, and in practical terms deal with heterogeneity.
The impact of heterogeneity on diagnostic accuracy
Concerns are apparently well founded. In a recent publication, Stålhammer et. al. [1] demonstrated that manual stratification of breast cancer patients into Luminal A vs. Luminal B based on Ki67 was associated with an error rate of 31%. Using image analysis with computer identified hot-spots reduced the error rate to 19%, when using PAM50 as a pseudo gold-standard.
Heterogeneity and hot-spots
What really made the difference in reducing error rates, was automated identification of hot-spots, using image analysis. Gudlagsson et. al [2] showed that as much as 50% of pathologists were unable to correctly identify the hottest hot-spot which, in some cases, can represent a major cognitive challenge. This challenge was effectively mitigated using image analysis.
Diagnostic applications
As a first practical diagnostic application, we considered automated hot-spot identification for Ki67 expression in breast cancer. By creating heat maps of biomarker expression, the intended use of the APP is to support pathologists in both visualizing heterogeneity and locate the hottest hot-spot (s). This can be used as input to APPs that quantify biomarker expression in the hot-spot(s). CE-marking for In-Vitro Diagnostic purposes, required special attention to the study designs for validating clinical performance.
Research applications
The challenges related to heterogeneity may well be further amplified when interrogating far more complex, and sometimes multiplexed, biomarkers across the entire tumor micro-environment. With Oncotopix® Author, the ability to visualize and quantify heterogeneity wrt. biomarker response has been generalized for tissue-based cancer research.
Click here to learn more about the CE-IVD Hot Spot APP.
Download the Hot Spot brochure
References
Michael Grunkin Categories: Blog 6260 Akoya Biosciences as an Authorized ResellerVisiopharm Announces Akoya Biosciences as an Authorized Reseller of Oncotopix® Discovery and Biotopix™
Visiopharm A/S announced today a partnership with Akoya Biosciences, the technology leader in multiplexed immunofluorescence including the Phenoptics™ portfolio with the Vectra® and Vectra Polaris® systems. As part of this agreement, Akoya becomes an authorized reseller of Visiopharm’s suite of image analysis software, including Phenomap™ and the teach-by-example Artificial Intelligence (AI) modules.
“Visiopharm is very pleased to have Akoya Biosciences join our partner and authorized reseller community. Our complementary technologies enable scientists and pathologists to better interrogate the disease biology through biomarker discovery and automated cell phenotyping within tissue samples. Our joint customers will benefit from two companies who are committed to driving innovation and providing solutions that deliver efficiency, standardization, and the reproducibility required to support large scale translational studies from discovery through clinical research” said Amanda Lowe, Senior Vice President of Visiopharm.
Akoya recently announced the acquisition of the Phenoptics portfolio from PerkinElmer, Inc., to complement the CODEX® platform for ultra-high multiplex capabilities. The enables analysis of multiplexed image data from the Mantra®, Vectra and Vectra Polaris platforms with future development for the CODEX technology.
“Akoya’s focus is to provide customers with end-to-end solutions for high parameter tissue analysis that includes multispectral imaging instruments, reagents, and powerful software.” said Terry Lo, President at Akoya Biosciences. “We are excited to be able to add software like Visiopharm’s Phenomap to our current portfolio and offer Akoya customers the best and broadest solutions in the field of multiplexed immunofluorescence and tissue analysis.”
The partnership with Akoya will cover both North America and Europe and support our joint vision of providing customers with a full suite of powerful image analysis solutions for high-dimensional, multiplex tissue assays.
Visiopharm will present Phenomap, AI, and our infinitely configurable suite of image analysis software at Pathology Vision’s (booth #214) in San Diego on November 5th and 6th and at Society of Immunotherapy in Cancer (booth #223) on November 8th – 11th in Washington DC.
Visiopharm Categories: Press Releases