Straticyte™ – A digitally delivered oral cancer predictor – powered by Visiopharm analysis

Kenneth P.H. Pritzker
M.D., BSc (Med), FRCPC
Dr. Ken Pritzker served as Chief, Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto from 1986 to 2008 and as a founder of Mount Sinai Services, a laboratory service outreach vehicle for the biomedical research and innovation sector.
With over 290 scientific publications and 27 book chapters, Ken is a recognized leader internationally in the fields of arthritis, biomaterials, genomics, cancer diagnostics and nanotechnology. Throughout his career, Ken has served in leadership and advisory positions to national and international professional societies, research institutes and healthcare organizations.
Ken Pritzker co-founded the company Proteocyte AI in 2011. The company just brought to market their first prognostic LDT test for oral pre-cancer lesions, Straticyte.
Visiopharm: Can you tell us more about your company Proteocyte and how it all began?
Dr. Ken Pritzker: Sure, it all started several years ago, when Dr. Ranju Ralhan and Michael Siu discovered, that the nuclear accumulation of S100A7 could serve as a predictor of poorer prognosis for Head and Neck squamous cell carcinoma and could predict a higher risk of transformation of oral premalignant lesions.1 We founded Proteocyte to dive deeper into this promising prognostic biomarker. Even though this finding could be applied to different cancer types, oral cancer was of particular interest to us, due to its high mortality of 50%, which is largely related to late diagnosis. Those late-stage lesions are very costly and difficult to treat. A high percentage of these cancers develop over multiple years in patients who have atypia initially, so they get a biopsy, but it often does not reveal pathologic dysplasia in the histopathologic examinations. Also, the histopathological dysplasia grading, which shows high inter- and intraobserver variation, does not relate very well to the risk of developing cancer from the lesion.2,3 However, some of those samples with mild dysplasia do show the S100A7 accumulation and thus have an elevated Straticyte value. So this is a very important finding because it alerts the clinicians to follow the patient fairly closely, despite their low histopathological grading.
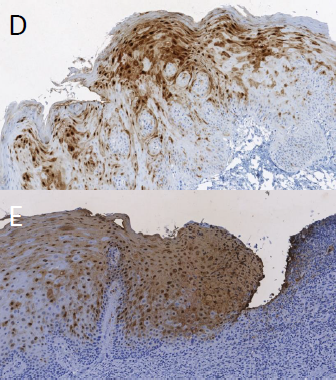
Figure 1 Immunohistochemistry staining of S100A7 biomarker in D) STRATICYTE Medium Risk E) STRATICYTE High Risk; From: BM Renick and MR Darling, Clinical Management of Patients With Oral Epithelial Dysplasia And Elevated STRATICYTETM Risk For Progression To Cancer, Poster @ IAOO, 2019
Visiopharm: What led you to choose Visiopharm and how has it been working with the company?
Dr. Ken Pritzker: We already knew the company and had worked with the software for several years back then at the Mount Sinai Hospital in Toronto. Already at this time, Visiopharm was one of the market leaders of morphometry applied to digital images of tissue slides. We already had a good and long relationship with them and the team, who provided advice. Back then it was just a few people, and we had a great relationship. They have been excellent, and the quality of support has been really superb, so we’ve been very happy with it. Visiopharm software is used to quantitate the immunohistochemical marker and it’s also used to measure certain morphological aspects of the cells. With the support of the Visiopharm team, we have created several APPs in Visiopharm, covering the whole workflow from region detection to score calculation.
Visiopharm: So how does Straticyte work and how can physicians use it?
Dr. Ken Pritzker: Our product Straticyte is an LDT test and it combines the evaluation of this specific IHC cell marker with an evaluation of cell morphometry. There are very few, if any, other biomarkers products out there which have a combination of two very different modalities. So this was an innovation. The other is that we saw ourselves from the beginning as an information company. The idea would be that the image of the oral epithelium would be transmitted to the company for analysis, and that we would have a serial database from which we could continue to develop the product and that has been a successful approach. Straticyte can calculate the 5-year probability that dysplastic lesions will progress to cancer. It has a sensitivity of 96% and a NPV of 95%, and those are significantly higher than the values for histological dysplasia grading, which are 75% and 59% respectively.4 The test is so good that if the Straticyte value is low, the patient can go back to live a normal life. But for cases with an elevated Straticyte value, those patients need to be watched closely and followed up against a possible recurrence or cancer. So compared to the current practice, Straticyte provides greater objectivity, sensitivity, and predictive power.
The way it works is that the physicians anywhere take a biopsy and that is assessed by the pathologist for whether there is pre-cancer present. If so, the pathology lab forwards unstained slides to one of our reference laboratories, which are contracted with Proteocyte and this lab performs the stains. The scanned image is then sent to us and we analyze the image and issue a report.
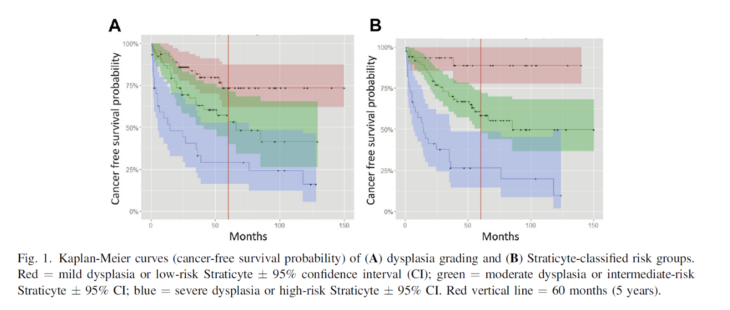
Figure 2: From: Hwang JTK et al, 2017, doi: 10.1016/j.oooo.2016.11.004
Visiopharm: How did you develop and validate your product?
Dr. Ken Pritzker: We started with samples from 250 patients with oral pre-cancer. From there we expanded to other sites in Canada, a site in Ireland and several sites now in the United States. By now, we have a database of more than 600 patients with clinical follow up. That’s probably the largest database of oral pre-cancer that exists, because typically, these patients present to local hospitals and if they have pre-cancer, they may not be followed up very well. So we know exactly the relationship between the test and the prognosis out beyond five years. It’s now more than ten sites and very different populations. And we noticed that lesions from different places have different characteristics. Those might reflect the state of health care in different places or the state of nutrition and this cannot be picked up if you do a single-site database.
At this point, the algorithm is very mature and our laboratory has received ISO15189 and CLIA accreditation. In the background we work on improving the tests, but it requires a considerable validation operation to move it from the version which we’re using, which is quite good to the next version, which might be a bit better.
Learn more about Straticyte.
——————————————————————————————————————–
Main references related to the interview
- Tripathi et al, 2010, DOI: 10.1371/journal.pone.0011939 ↩︎
- Dost F, Le Cao K, Ford PJ, Ades C, Farah CS. Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:343-352. ↩︎
- Fleskens S, Slootweg P. Grading systems in head and neck dysplasia: their prognostic value, weaknesses and utility. Head Neck Oncol. 2009;1:11. ↩︎
- Hwang et al, 2017, DOI: 10.1016/j.oooo.2016.11.004 ↩︎



