Visiopharm is pleased to announce that Johanne Louise Brændgaard has been appointed Chief Marketing Officer.
Johanne will play a vital part in executing Visiopharm’s strategy in AI-driven precision and high-throughput pathology, with responsibility for brand strategy and awareness, customer experience and commercial marketing.
Johanne brings extensive global experience of sales, marketing, and product management within the medical technology industry from previous positions at Cook Medical and Getinge. She also has experience from the telecommunications and venture capital industries.
Johanne is currently on the board of ADDvise AB, having previously been on the board of Acarix AB.
Johanne has a master’s degree in International Business Economics from Aalborg University in Denmark.
Michael Grunkin, Visiopharm CEO said:
“We are delighted to welcome Johanne. She is a great fit with the team, culture and values of the company and brings a personality with her that will naturally facilitate cross-functional collaboration. That, in combination with her extensive experience in the medical technology industry, will be a great asset as we continue to move forward with our ambitious marketing goals.”
Johanne Louise Brændgaard said:
“I’m excited to have joined Visiopharm at this critical time and be part of the mission to transform pathology through our AI-driven solutions. This will enable our customers to have a much stronger foundation for making diagnostic and treatment decisions for cancer patients, which in turn can have significant socio-economic impact due to lower rates of misdiagnosis and ineffective treatment.”
 Visiopharm
Categories: Press Releases
17989
Augsburg University Hospital partners with Visiopharm for digital transformation
Visiopharm
Categories: Press Releases
17989
Augsburg University Hospital partners with Visiopharm for digital transformation
Augsburg University Hospital, one of Germany’s leading hospitals, has selected Visiopharm, a world-leading developer of precision AI image analysis software, to realise its ambition of becoming a digital pathology powerhouse.
The partnership will see Visiopharm implement its digital pathology software to accelerate the hospital’s diagnostic workflows, to provide faster diagnoses to patients and even with a higher degree of accuracy.
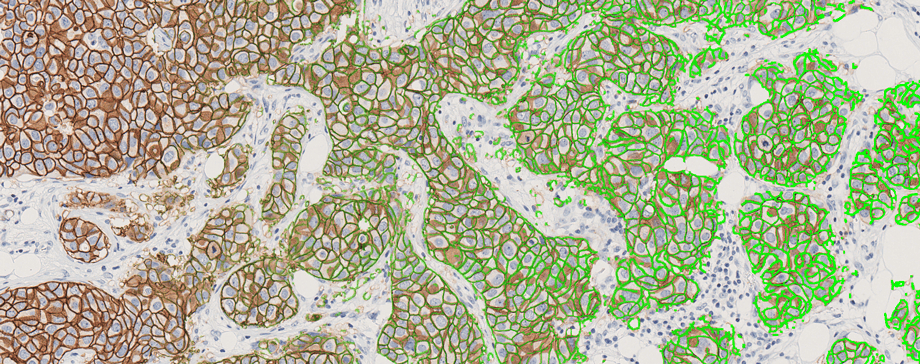
The hospital will be using Visiopharm’s CE-IVD marked APPs, starting with HER2 and then expanding to other APPs like ER, PR, Ki67 and lymph node metastasis detection to quantify breast cancer biomarkers.
The partnership builds on an existing collaboration between Visiopharm and Prof Ralf Huss, a member of Visiopharm’s Scientific Advisory Board and Head of the Center for Digital Medicine at Augsburg Hospital. In a collaborative paper published recently, Augsburg pathologists reported on the use of Visiopharm’s AI software to discover an impaired maturation of dendritic cells leading to an inadequate T-cell response in severe cases of COVID patients at the hospital.
Augsburg and Visiopharm intend to collaborate on demonstrating the value of introducing digital pathology and AI into the clinical workflow with the aim for Augsburg to confirm its role as a reference site for other hospitals to base best practice on. This will involve conducting scientific and rigorous studies, as well as developing and testing new algorithms for diagnostic decision support, stain quality management and slide control.
To ensure a seamless workflow, Visiopharm software will be integrated into the existing pathology workflow of the pathology department, together with NEXUS AG, which is the provider of the laboratory information system used in Augsburg.
Michael Grunkin, Visiopharm CEO said:
“We’re excited to be given this opportunity to help Augsburg University Hospital achieve their digital transformation ambition. It’s an exciting milestone to modernize their pathology services and show the value of a fully digital workflow in improving patient care. Throughout the project, we very much look forward to sharing what we achieve together with the healthcare community.”
Prof Bruno Märkl, Institute for Pathology and Molecular Diagnosis, Augsburg University Hospital said:
“This is the beginning of a new chapter for our hospital’s future. With Visiopharm’s AI software we can introduce a truly digital workflow, which includes diagnostic decision support and enables standardization of stain quality. The whole team is determined to deliver the best quality of care for our patients, and we cannot do this without digitizing our pathology workflow. Hopefully we can inspire other hospitals to follow in our footsteps.”
About Visiopharm
Visiopharm® is a world-leading developer of precision AI image analysis software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs support primary diagnostics. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 900 licenses and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, Netherlands and United States.
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
