Visiopharm has been granted the European patent, Method for labelling histopathological images and training deep learning models, EP3639191. The patent is still pending in the US and other jurisdictions.
For a relatively broad range of applications, the method is addressing one of the most significant bottlenecks in the development of new Deep Learning applications for pathology, which is obtaining training annotations. For such applications, the method will eliminate this rate-limiting, time-consuming, and costly step. An example of the utility of this method is published in Automated Quantification of sTIL Density with H&E-Based Digital Image Analysis Has Prognostic Potential in Triple-Negative Breast Cancers.
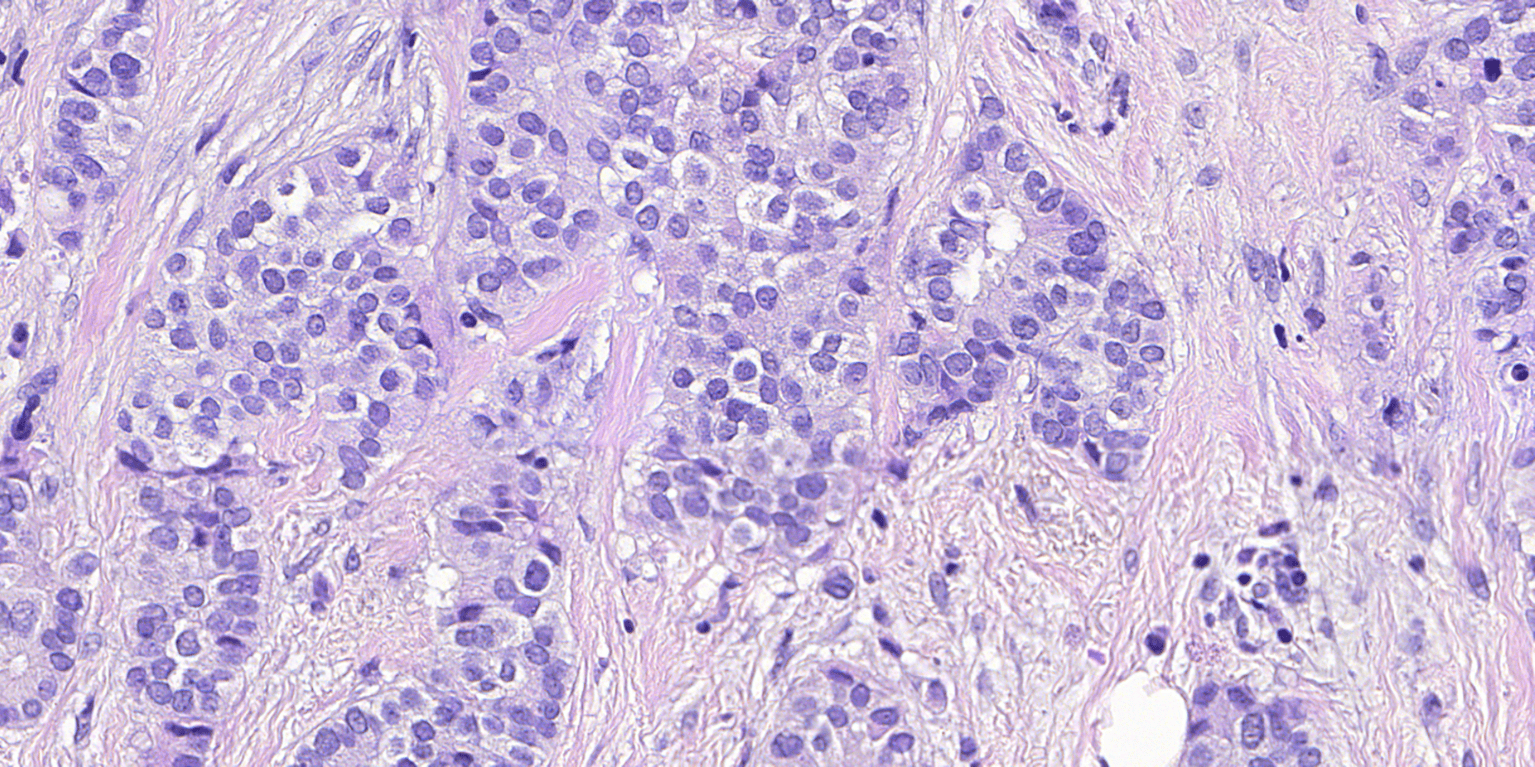
Whenever a Deep Learning Network is required to recognize certain structures in stained tissue sections, training typically relies on hand annotations by several experienced pathologists, sometimes followed by an adjudication session for cases with disagreement. Such datasets are inherently noisy, due to unavoidable inaccuracies, concomitantly requiring larger datasets to statistically ‘cancel out’ the noise components.
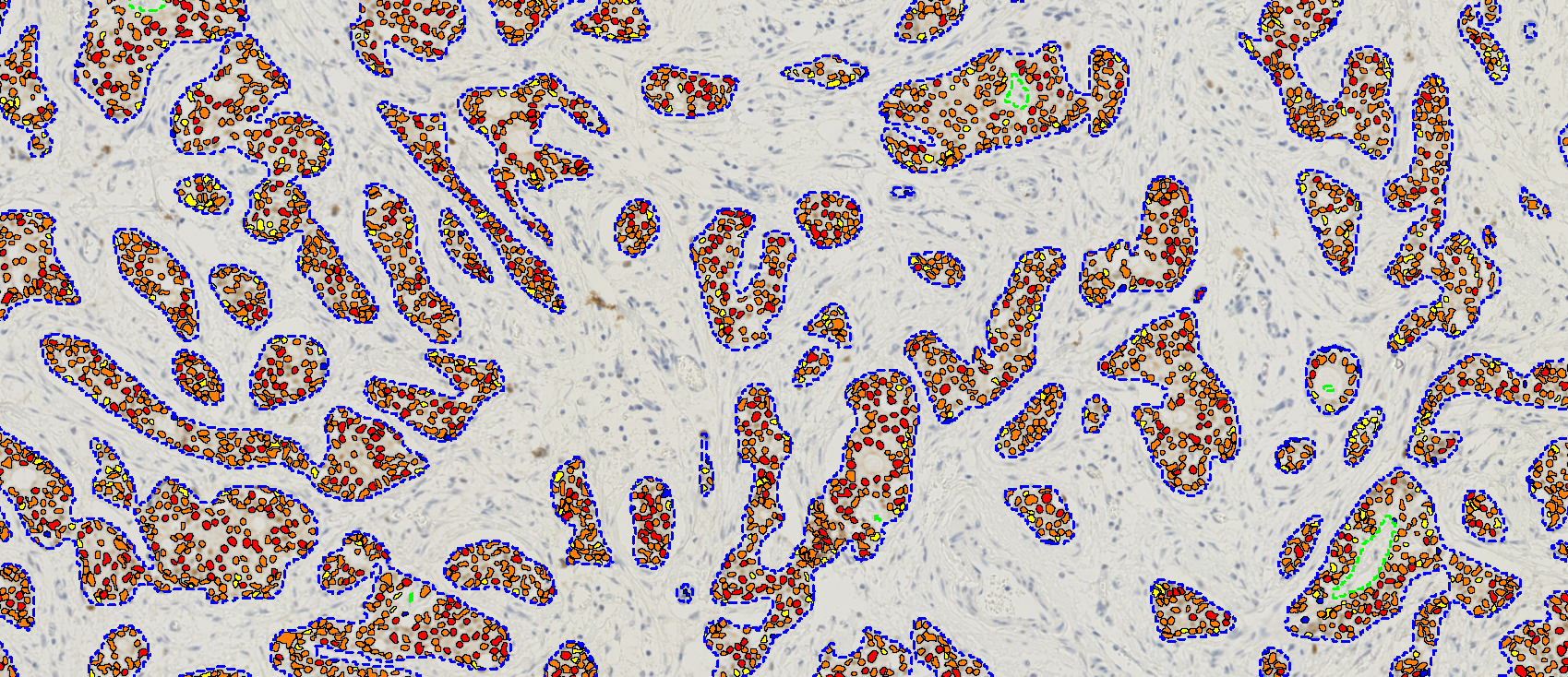
The method proposed here, can be used whenever the structures of interest can be labeled with IHC, whether it is BF of IF. Structures labeled in this way are typically very easy to segment with image analysis. The regions can then be transferred to either a serial section, or a serially stained section, where they are used as training annotations.
This critical step relies on Visiopharm’s global patent for Virtual Multiplexing method described in the European Patent Office and US, where two or more slides that can be aligned at very high speed and accuracy.
Jeppe Thagaard, head of AI, Visiopharm said:
“This method does not only lower the cost and time of manual annotation, but it also allows us to create training data sets with much higher annotation consistency – one of the most important aspects of developing deep learning-based applications. This, in turn, has the potential to greatly reduce the volume of training data required to achieve sufficient clinical performance of the DL network.”
Johan Doré, CTO, Visiopharm said:
“This is the kind of breakthrough we need to dramatically accelerate the creation of diagnostic decision support- and quality control content for our Image Analysis Management System. The Oncotopix IAMS is scanner agnostic, and also plugs agnostically and directly into any digital pathology workstream, whether it is driven by a LIMS or a PACS.”
 Visiopharm
Categories: Press Releases
15500
Visiopharm and Agilent expand collaboration with worldwide distribution agreement
Visiopharm
Categories: Press Releases
15500
Visiopharm and Agilent expand collaboration with worldwide distribution agreement
Agilent Technologies Inc. (NYSE: A), a leader in life sciences, diagnostics, and applied chemical markets, and Visiopharm, a leader in AI-based digital pathology, today announced the signing of a worldwide distribution agreement, enabling Agilent to co-market Visiopharm’s portfolio of CE-IVD marked artificial intelligence (AI)-driven Precision Pathology software in addition to Agilent’s portfolio of automated pathology staining solutions. With an initial focus on Europe, this agreement marks Agilent’s first entry into the growing digital pathology market and strengthens the relationship between the two companies by further expanding the scope of their long-term collaboration.
Digital pathology is experiencing rapid growth worldwide, with its adoption expected to double by the end of this decade. As the use of digital pathology increases, AI-driven pathology solutions are emerging as the new standard. These solutions can provide diagnostic decision support and productivity enhancements that enable pathologists to improve diagnostic accuracy and workload management.
This adoption has been accelerated by the COVID-19 pandemic, which has found pathologists increasingly reviewing cases remotely. In the United Kingdom, for example, pathologists have received guidance for remote reporting of digital pathology slides in times of exceptional service pressure, and support in the adoption of technologies, such as digital pathology, that have the potential to aid in their assessment.
“This is an exciting next step in our partnership with Visiopharm,” said Lou Welebob, vice president and general manager of Agilent’s Pathology Division. “Being able to offer our customers digital pathology software alongside our range of automated staining solutions is a powerful combination that can support pathologists in their assessment and help improve patient care. We look forward to our continued collaboration with Visiopharm as we work together toward the fully digitalized pathology lab of the future.”
Michael Grunkin, CEO, Visiopharm said: “We’re delighted to further expand our partnership with Agilent. With strong complementary solutions, we will be able to address recognized and currently unmet needs for both standardization and automation. This distrubution agreement is a logical first step towards supporting those labs who share our joint vision of automated AI-driven Precision Pathology.”
Prior to this announcement, Agilent and Visiopharm have collaborated to validate Visiopharm’s existing HER2 APP to include Agilent’s new HercepTest mAb pharmDx for Dako Omnis, both CE-IVD marked and available for sale in Europe. Released in October 2020, this product enables pathologists to use the Visiopharm HER2 APP for objective decision support in the assessment of HercepTest™ mAb pharmDx stained slides, saving valuable time for both pathologists and patients.
For in vitro diagnostic use in Europe.
HercepTestTM mAb pharmDx (Dako Omnis) (Code GE001):
The licensed antibody is created by Epitomics Inc. (an Abcam company), using Abcam’s proprietary rabbit monoclonal antibody technology covered under Patent No’s 5,675,063 and 7,402,409.
HercepTest™ and Herceptin® are trademarks owned by Genentech, Inc.
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
