We are an exclusive distributor of Hamamatsu’s NanoZoomer whole slide scanners in the Netherlands and Denmark. Hamamatsu fuses cutting-edge hardware technology with the philosophy of intuitive interface design. As a result, the NanoZoomer series offers high productivity and user acceptance within multiple whole slide imaging application environments.
We are a leading provider of AI-driven precision pathology software
We are a leading provider of AI-driven precision pathology software for research and diagnostics. In research, we are a technology leader providing tools that help scientists, pathologists, and image analysis experts produce accurate data for all types of tissue-based research. In diagnostics, we are a leader within clinical applications, with no fewer than nine diagnostic algorithms cleared under IVDR for EU customers. These applications provide diagnostic decision support and can be easily activated and integrated into existing lab workflows.
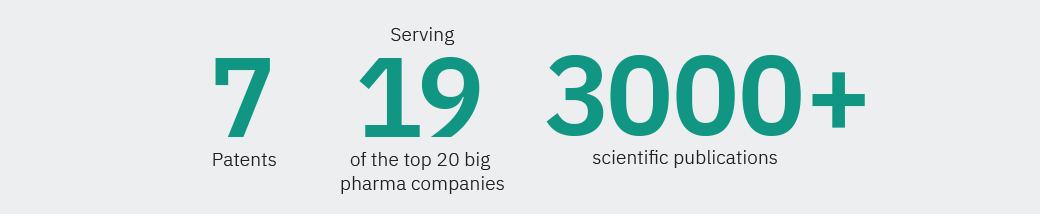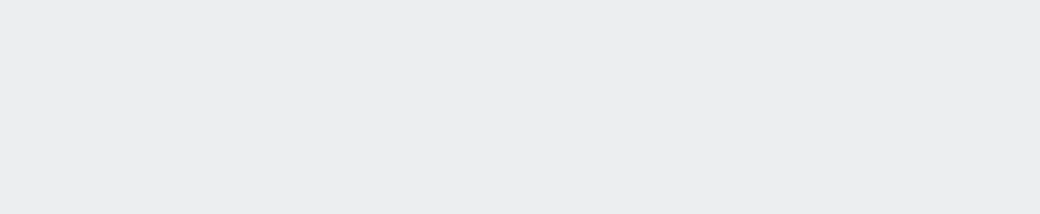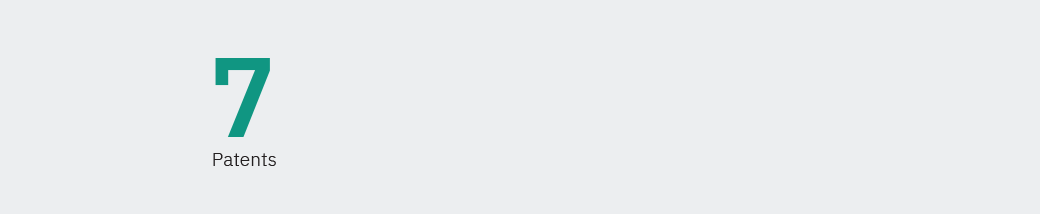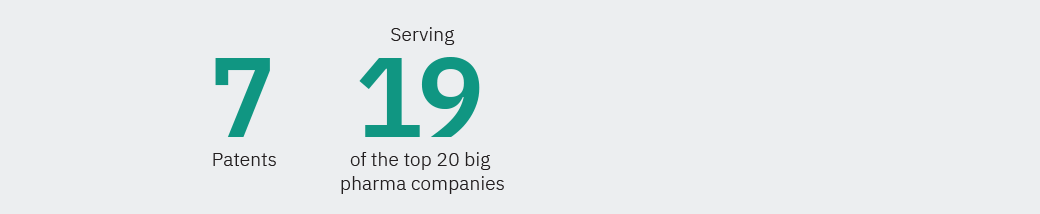
Founded in 2002, we are privately owned and operate internationally with over 750 customer accounts in more than 40 countries. Our company’s headquarters are located in Denmark’s Medicon Valley, with legal entities in Sweden, the UK, Germany, the Netherlands, and the United States, and local representation in France and China.
Privately-owned company
We are a privately owned company, founded in 2002 by our Managing Director and Chief Executive Officer, Michael Grunkin, and Chief Technical Officer, Johan Doré Hansen. Both Grunkin and Hansen have a strong scientific and practical background in image analysis.

Leading scientific advancement
Our solutions have been featured in over 3,300 scientific publications. We are certified according to EN ISO 13485:2016, covering the design, development, manufacture, installation, and service of in vitro diagnostic pathology software.
Technology & AI
Our company has grown into an international business with over 750 accounts in more than 40 countries. Our growing network of authorized distributors and partners support the growth of Visiopharm’s solutions on several continents including North America, Europe, and Asia.

Our history
Awards and Certificates

Frost & Sullivan – Global Digital Pathology Solutions 2019
Visiopharm Earns Acclaim from Frost & Sullivan for Transforming Anatomic Pathology. Frost & Sullivan recognizes Denmark-headquartered Visiopharm A/S with the 2019 Global Company of the Year Award. The award recognizes a high degree of innovation with products and technologies, and the resulting leadership in terms of customer value and market penetration.

CIO Applications Top 25 Machine Learning Solution Providers 2019
Visiopharm was recognized by CIO Applications Magazine as CIO Applications Top 25 Machine Learning Solution Provider 2019. CIO Applications brings you the “Top 25 Machine Learning Solution Providers – 2019”. The CIO Application Magazine states: “The list features some of the most prominent machine solution providers in the industry that have excelled in their services and product portfolio in the ML space”.

Børsen Gazelle Growth Accelerator Award
Visiopharm wins Denmark’s leading business newspaper “Dagblade Børsen” Gazelle Growth Accellerator, the prestigious award “Børsen Gazelle 2017” as an identification of Denmark’s absolute growth elite.
The award is given as a recognition to achieve a continuous growth in revenue or gross profit for the last four financial years, and which has, in total, more than doubled the revenue or the gross profit in the period.

Danish EY Entrepreneur Of The Year™
Visiopharm was promoted two times for EY Entrepreneur of the Year in 2016 and 2017. We were placed among the top three nominees for the finalist in the Life Science category in 2017.
Ernst & Young, EY Entrepreneur Of The Year™ awards recognize and celebrate unstoppable entrepreneurs in their field.








