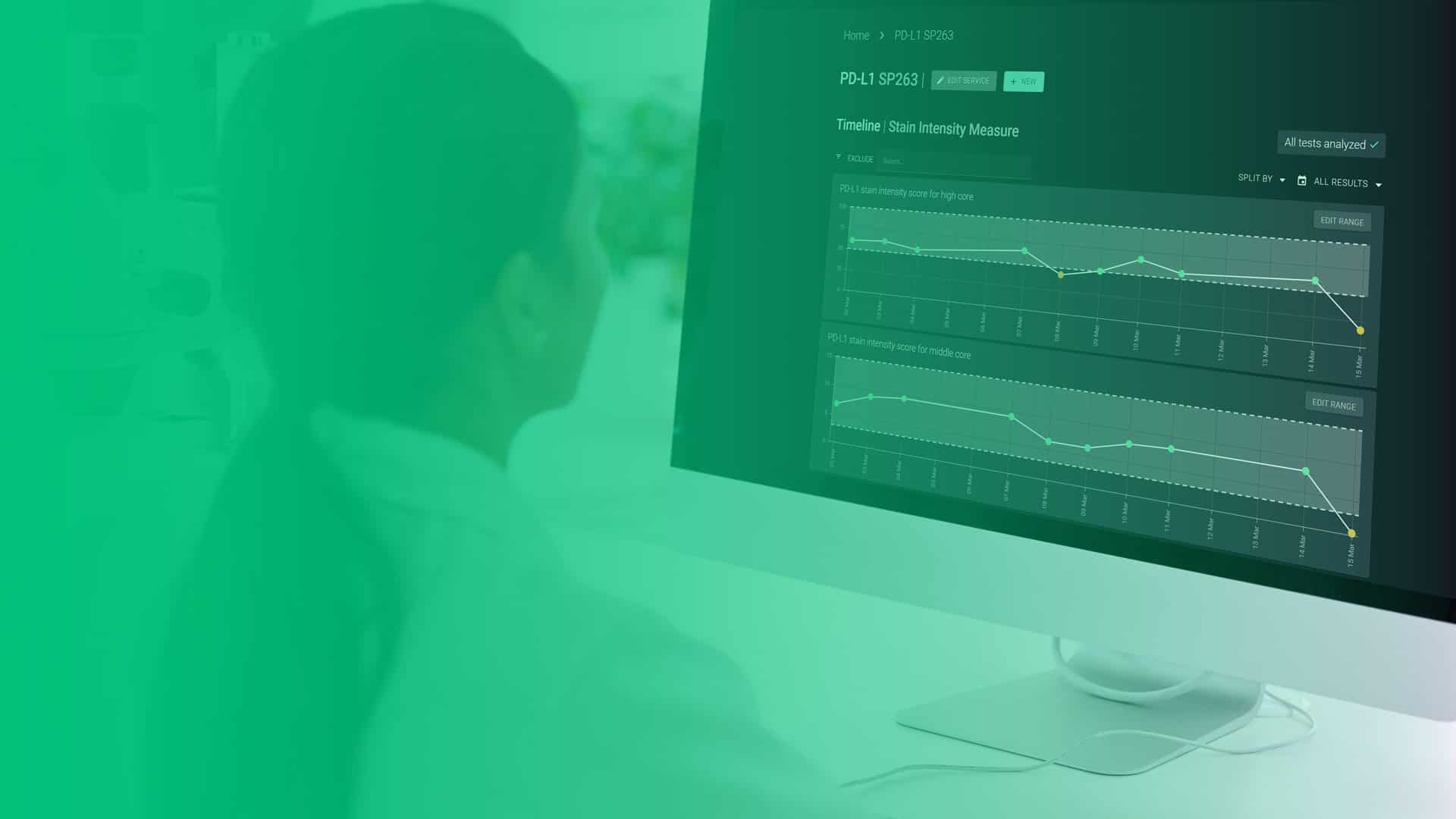
Measure and document your staining consistency through standardized analysis of next generation reference materials
Trust your staining through AI driven quantification and monitoring
Get started easily
with a frictionless set up and intuitive workflow
Be prepared
for new demands and regulations
10x higher error rate in IHC
For decades, the reported error rates of IHC staining have been 10 times higher than error rates for other clinical tests, risking or causing wrong diagnosis. There is an ongoing push for more stringent regulations to ensure the highest quality of diagnostic decisions.
Learn more about the underlying problems and how recent publications have been unveiling the root causes.
Challenges in IHC staining“Thanks to the feedback we got, we were able to stabilize important markers like HER2 and PD-L1, and could solve some issues with our stainers.”
Sven van Kempen, Research Analyst, UMC Utrecht, The Netherlands

Trust your staining through AI driven quantification and monitoring
Qualitopix allows you to measure your staining intensity daily and monitor the measurements through a Levey-Jennings plot. Any variation from the norm is immediately flagged, so instant action can be taken. Using next generation standardized controls in conjunction with AI quantification enables you to objectively measure the consistency of your assays.
Selected examples of issues detected by Qualitopix
Bad reagent lot
Levey-Jennings chart of multiple HER2 runs over time. A sudden rise of intensity values was detectable for all the cell line cores and could be linked to a specific reagent lot number. Switching the reagent lot allowed the staining to return to normal.
Positional inconsistencies
Single run of PD-L1 with intensity values shown for each position in the stainer rack. A drop in intensity is visible for positions 21-29. After investigation the reason for this was identified as an incorrect leveling of the slide rack.
Insensitive HER2 clone
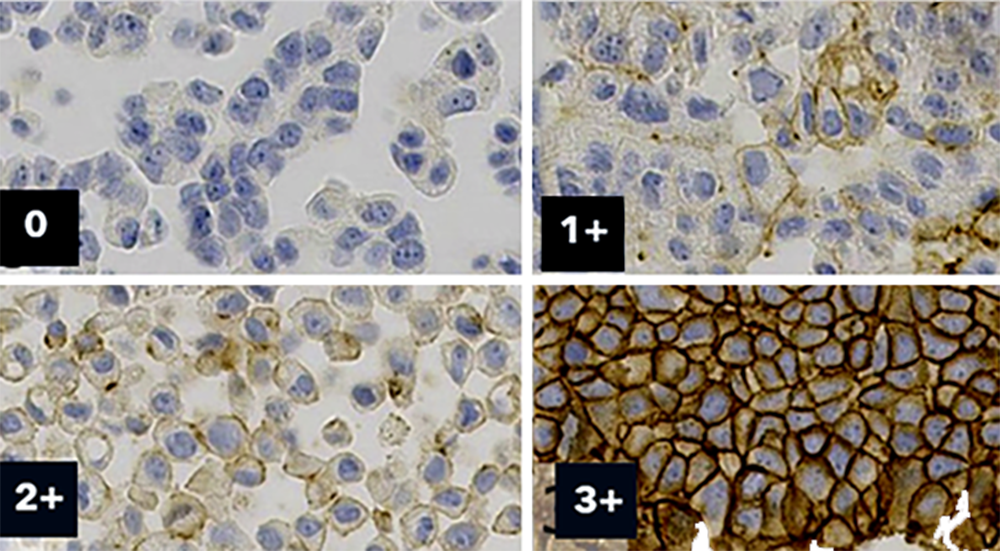
Multiple HER2 runs over time on two stainers of the same type. No signal on the 1+ and 2+ cores and weaker than expected 3+ core. Using a different clone on both instruments showed expected signal.
We collaborate with






Get started easily with a frictionless set up
and intuitive workflow
No local installation or integration, an intuitive workflow and a fast turnaround time make it easy for you to add Qualitopix to your workflow.
Ready to use cell lines with standardized biomarker expression are added to a slide, scanned and uploaded to Qualitopix.
No scanner available?
We have a low-cost single slide standalone scanner (Grundium) available on demand.
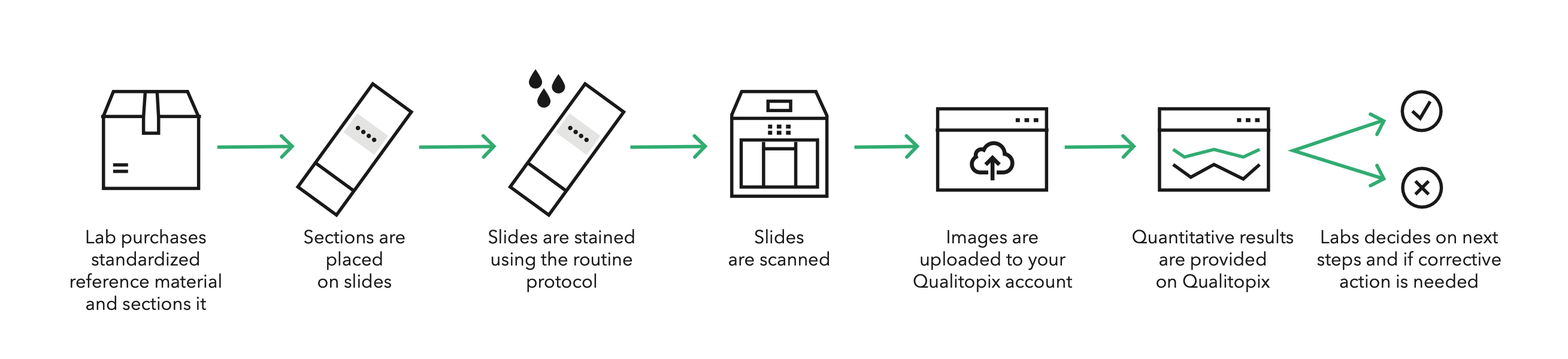
Purchase standardized reference material
Cell lines (Histocyte, Array Science)
Coming soon: Microbead controls and calibrators (BCS)
Place on slides and stain
Follow your routine staining protocol.

Scan slides
Slides can be scanned on various scanners. If needed, we have a low cost / volume scanner optionally available.
Upload images and metadata
No installation required. Just drag and drop the images with key metadata.
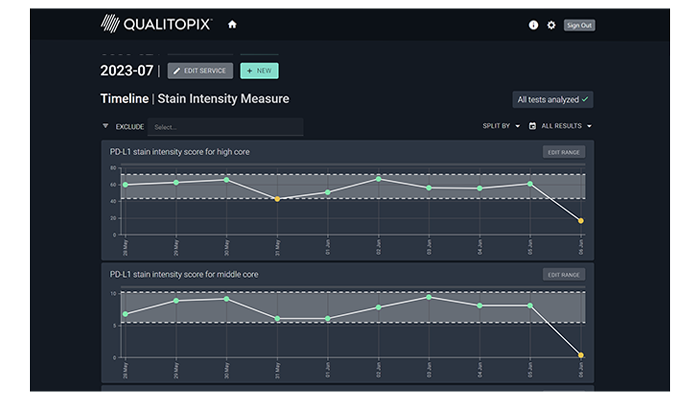
Receive quantitative results
Shortly after upload, you will receive results and will be notified if a test requires your attention. Qualitopix provides tools to support your troubleshooting process.
Be prepared for new demands and regulations
There has been a recent change to the ISO 15189:2022 regarding use of third-party controls as well as monitoring trends and statistics for stain assays. The documentation tools in Qualitopix allow to you comply with the new regulation.
More change is in the air, since Barbarajean Magnani and Clive Tayor published their editorial “Immunohistochemistry Should Be Regulated As an Assay”, (full paper) where they demand the same checks and regulations as already applied in other laboratory assays to lower the error rate. The discussion has started and might cause more regulations to come into effect.
Additionally, novel markers and new Companion Diagnostics will emerge. The recent focus on correct scoring of Her2-low patients has raised the question, if the staining in the labs enables consistent differentiation between HER2 0 cases and HER2 1+ cases.
Sign up to access our interactive online demo
After registration, you will receive a link to login into our demo version of Qualitopix. Review example staining results and see how Qualitopix allows you to monitor your stain consistency.