If you work with biomarker analysis, you’ll appreciate that there is potential for error every step of the way – from getting the sample to accurate staining. For research results and patient care alike, this can have significant consequences.
How consistent is your tissue staining?
And how certain are you about it?


The clinical utility of tissue biomarkers is linked to the assay’s analytical sensitivity and its reproducibility. No matter if used in diagnostics or clinical studies, the tissue staining and resulting reporting should not depend on which lab processes the sample. While participation in EQA runs is important for comparing inter-lab consistency and can improve staining performance, those tests run only 1-2 times a year.

“Precision medicine demands precision pathology. IHC from a Stain to an Assay – the TIME is NOW.”
Watch the webinar from Clive Taylor and learn, why IHC should be regulated as an assay and not as just a stain.
Clive Taylor webinarThe reported error rate in IHC is significantly higher than the <1% for other laboratory tests and Dr. Steve Bogen identified different reasons for this in his root cause analysis4:
No usage of standardized control material
Those are often produced inhouse, of various tissue types and biomarker expression and can be heterogenous.
No possibility to measure absolute sensitivity
There has been no information, if and to what limit the assay can detect low expression levels and how this compares to other labs.
No quantitative monitoring of
assay performance
Without regular measurements, slight shifts in staining performance cannot be monitored and fails will go undetected.
New approach to measure biomarker detection limits discovers 10fold wide range of sensitivity in paper3 by Emina Torlakovic.
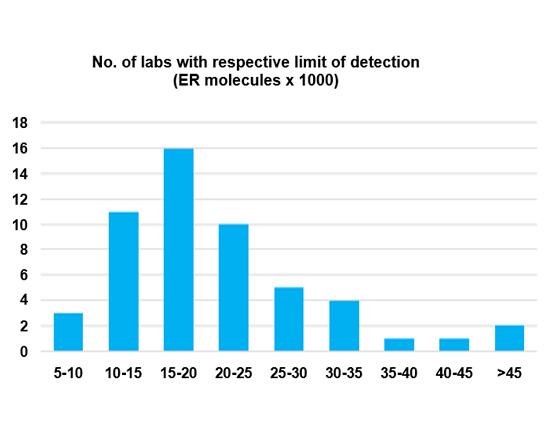
Range of sensitivity
A wide range of sensitivity (= Lower Limit of Detection) was detected across 53 Canadian labs using the same antibody clone for ER IHC.
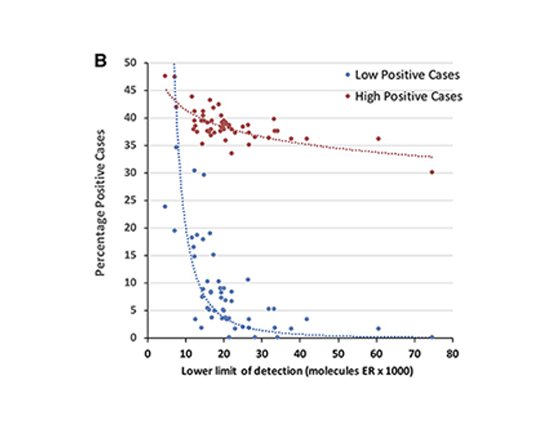
Problems with ER-low detection
Percentage of all positive cases (high and low) in correlation to the analytic sensitivity.
The higher the lower limit of detection (=low sensitivity), the less positive ER cases are being identified, especially affecting the low positive cases.
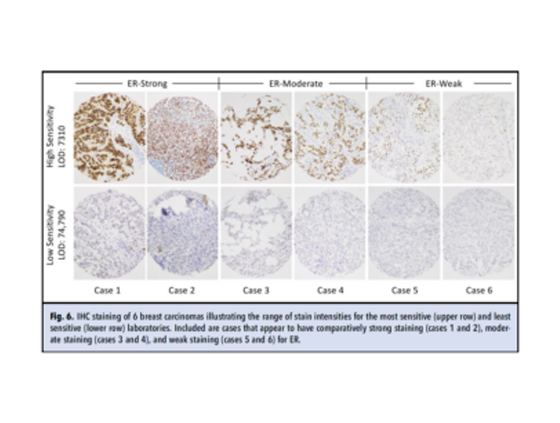
Range of stain intensity
IHC staining of 6 breast carcinomas illustrating the range of stain intensities for the most sensitive (upper row) and least sensitive (lower row) laboratories. Included are cases that appear to have comparatively strong staining (cases 1 and 2), moderate staining (cases 3 and 4), and weak staining (cases 5 and 6) for ER.
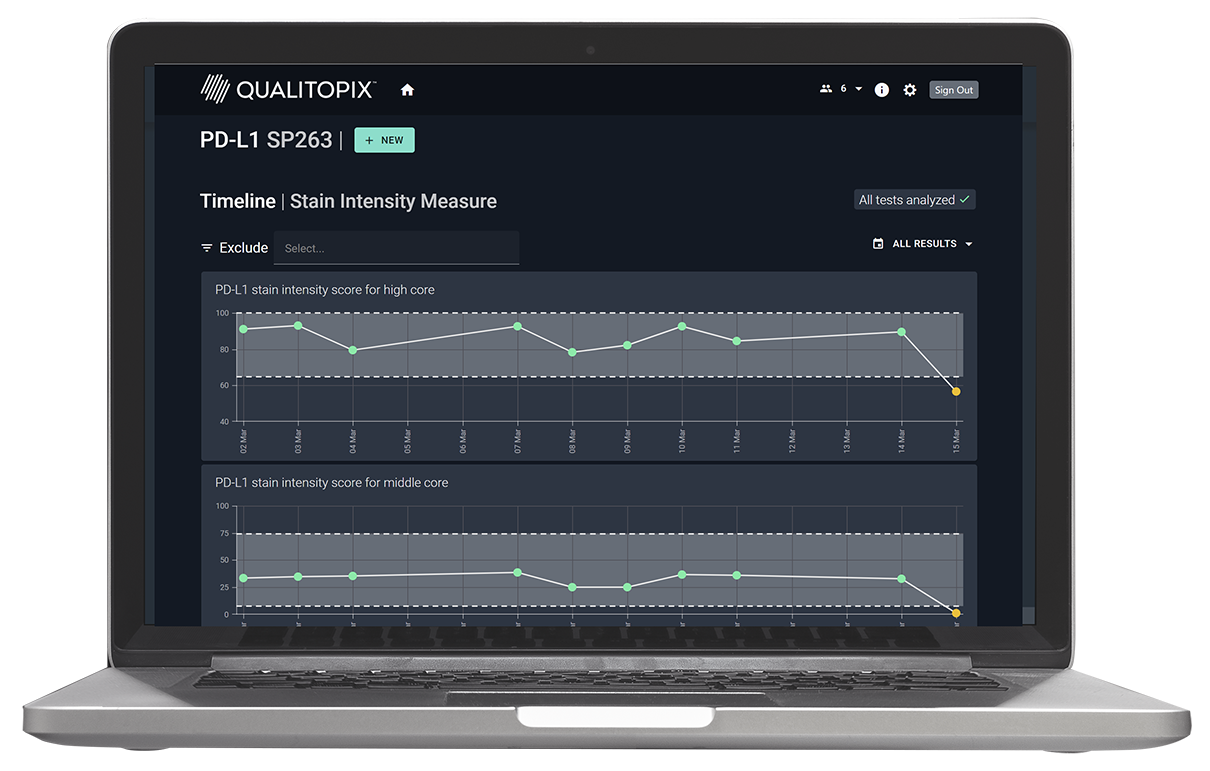
Introducing Qualitopix®
Measure and document your staining consistency
References
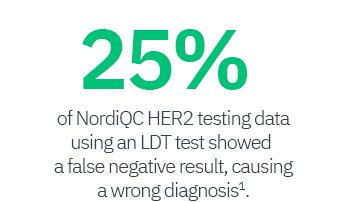
1 Vyberg M et al. Immunohistochemical expression of HER2 in breast cancer: socioeconomic impact of inaccurate tests. BMC Health Serv Res., 15:352, August 2015 Read full paper
2 Nielsen S et al., Lessons Learned, Challenges Taken, and Actions Made for “Precision” Immunohistochemistry. Analysis and Perspectives From the NordiQC Proficiency Testing Program. Applied Immunohistochemistry & Molecular Morphology, 31(7):p 452-458, August 2023 Read full paper
3 Torlakovic E et al., Development and Validation of Measurement Traceability for In Situ Immunoassays, Clinical Chemistry, 67(5):763–771, May 2021 Read full paper
4 Bogen SA. A Root Cause Analysis Into the High Error Rate in Clinical Immunohistochemistry. Appl Immunohistochem Mol Morphol., 27(5):329-338, May/Jun 2019 Read full paper

