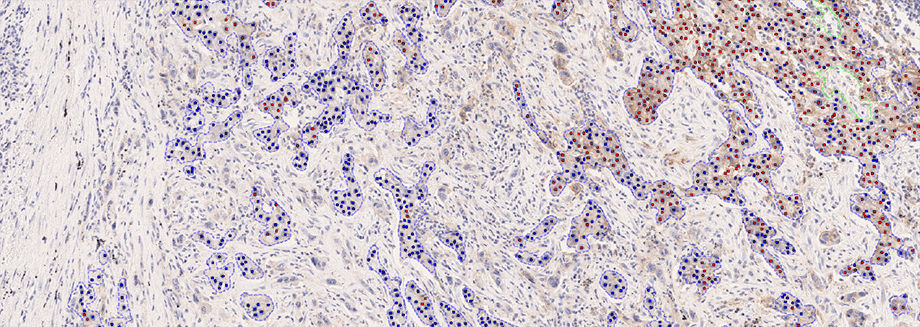
Hørsholm, Denmark — Visiopharm A/S, an innovator in AI-powered digital pathology solutions, has announced the IVDR certification of its PD-L1 APP for non-small cell lung cancer (NSCLC). The APP provides fully automated evaluation for the predictive biomarker, offering whole slide analysis results to the pathologist with no prior interaction needed. With exceptional accuracy and consistency in biomarker scoring related to eligibility for immune checkpoint inhibitors, it integrates seamlessly with PACS/IMS systems used by pathology labs, ensuring optimal workflow efficiency.
The APP is the first IVDR-cleared PD-L1 algorithm in the market and expands Visiopharm’s existing diagnostic portfolio for breast, colorectal, and prostate cancer with lung cancer – the second most common cancer type but with the highest number of deaths per year [1]. The product addresses the needs of the current diagnostic market where prescriptions of certain PD-L1 immune checkpoint inhibitors have grown more than 500% between 2020 and 2021 alone [2]. With a growing technology stack for precisely quantifying predictive biomarkers, it shows Visiopharm’s leading capabilities to support the needs of pharma for precision pathology.
Achieving IVDR requires a significant effort, and the PD-L1 APP is the 9th IVDR clearance for Visiopharm. The validation is based on the results of a comprehensive clinical study encompassing a large patient cohort and evaluations from three distinct European sites. The study directly compared manual assessments with the APP’s stand-alone analysis and APP-assisted interpretations. The results were remarkable, demonstrating substantial to near-perfect agreement with the multi-reader reference score and a high concordance with gold-standard expert manual assessments. Most notably, the data indicates increased agreement rates with pathologists reporting efficiency gains when using the APP, underpinning the fully automated APP’s potential to address the primary challenges of PD-L1 IHC scoring.
Professor Ralf Huss from University Hospital Augsburg, Germany, reflects on the APP’s impact: “After getting acquainted with the APP and building trust in the scores, it was much easier and faster to score PD-L1 NSCLC. It took less than 20 cases doing a site-by-site comparison of the manual scoring with the APP-assisted scores to build up that level of trust. The possibility to interact with the classification and review the image also allows for a very quick plausibility check, if there is some doubt on the APP-generated results, e.g. does the APP only score tumor cells, etc. Now I don’t want to miss the APP anymore to do PD-L1 NSCLC scoring, especially on a larger scale.”
Visiopharm has partnered with Agilent Technologies Inc. to develop and validate the PD-L1 Diagnostic APP adding to its expansive portfolio of fully automated workflow APPs that have been validated using Agilent’s Dako Omnis platform.
Lou Welebob, Vice President and General Manager, Diagnostics and Genomics Group, Agilent comments, “The IVDR certification of Visiopharm’s PD-L1 APP for non-small cell lung cancer (NSCLC) represents a significant advancement in digital pathology. We are excited about the algorithm-assisted advances in pathology scoring and to see how workflow integration may deliver on overall efficiency.”
Dirk Vossen, CDO of Visiopharm, emphasizes the company’s commitment: “Our PD-L1 algorithm exemplifies our drive to refine diagnostic accuracy and support improved efficiencies. Furthermore, it is a product of our fruitful alliance with Agilent.”
See more information on the APP in our APP Center
About Visiopharm
Visiopharm® is a leading provider of AI-driven precision pathology software for research and diagnostics. In research, it is a technology leader providing tools that help scientists, pathologists, and image analysis experts produce accurate data for all types of tissue-based research. In diagnostics, it is a leader within clinical applications, with no less than nine diagnostic algorithms cleared under IVDR for EU and UK customers. These applications provide diagnostic decision support and and can be easily activated and integrated into existing lab workflows.
Founded in 2002, Visiopharm is privately owned and operates internationally with over 750 customer accounts in more than 40 countries. The company’s headquarters are located in Denmark’s Medicon Valley, with offices in Sweden, the UK, Germany, the Netherlands, and the United States, and local representation in France and China.
[1] WHO February 2022 (www.who.int)
[2] Shin, Y. ., Kumar, A. & Guo, J.J. Spending, Utilization, and Price Trends for Immune Checkpoint Inhibitors in US Medicaid Programs: An Empirical Analysis from 2011 to 2021. Clin Drug Investig 43, 289–298 (2023)
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
