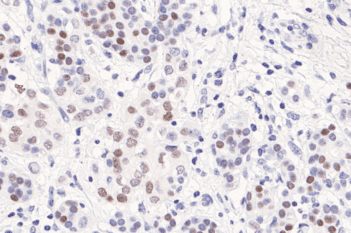
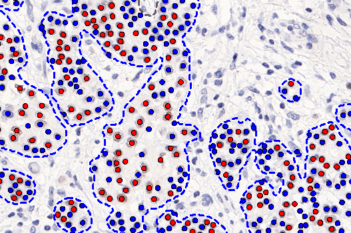
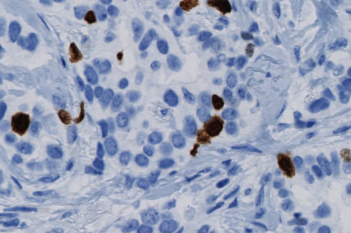
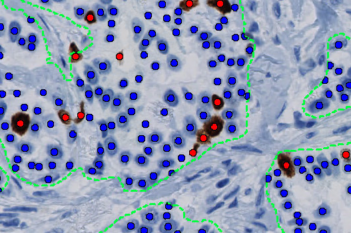
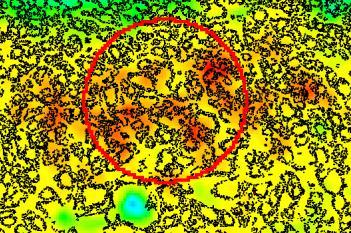
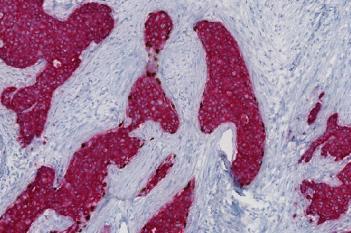
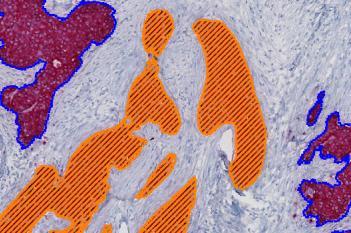
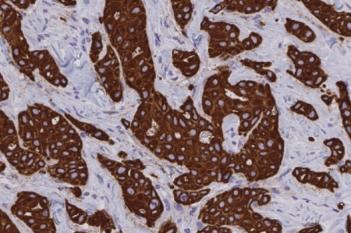
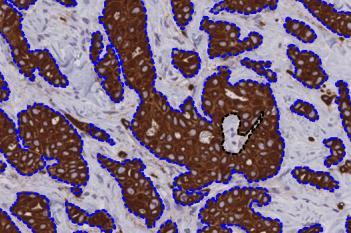
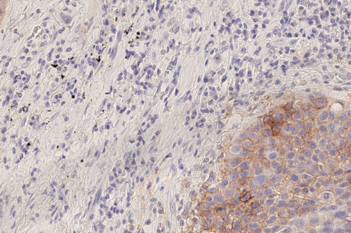
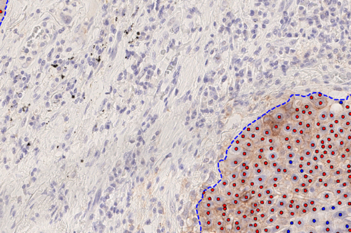
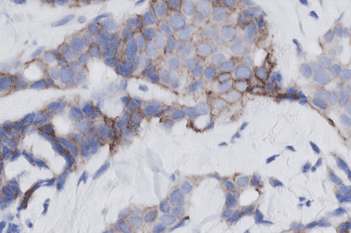
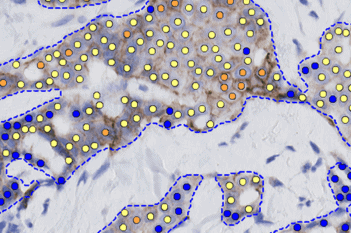
Our IVDR certified APPs deliver reliable and consistent biomarker quantification across different cancer types, so you can stop counting – freeing up time for you and your lab.
Fully automated zero-click workflows –
integrated into your existing setup.
Let our AI do the counting. Focus on the diagnosis.
Pathologists today face growing challenges, balancing high diagnostic demands with limited time and increasing case volumes. The growing complexity of the biomarker scores requested for the new cancer therapies is challenging the capabilities of the human eye, but accurate and consistent scores are needed for today’s precision medicine.
Accuracy for patients
Enhanced sensitivity on prognostic and predictive biomarkers could improve patient outcomes, minimizing false negative cases, who would otherwise be denied treatment.
Confidence for pathologists
Our APPs deliver higher accuracy and reproducibility than the human eye is capable of. Stop eye-balling and measure exactly how close you are to the cutoffs. Without a doubt.
Efficiency for labs
Proven higher sensitivity for lymph node metastasis or HER2 scoring can save potential IHC stains or reduce reflex testing. This saves time for you and your lab, that can be used elsewhere.
The largest portfolio of IVDR certified APPs
Diagnostic decision support
Our CE-IVD APPs are for in vitro diagnostic use in EU/UK. They are not for sale in the USA, where we have respective translational APPs available.
Show all CE-IVD APPs
[AI] allows for better and much more accurate reading of known biomarkers, which, despite the development of new staining techniques, remains challenging.”
Professor Torben Steiniche, MD, Department of Pathology, Aarhus University Hospital, DK
Precision that pays off
We know, that going digital is a marathon, financially as well as effortwise. Adding AI-based analysis solutions can pay you back by save reviewing time, while even increasing the sensitivity. This is not just pathologist’s time, it is also freeing up valuable lab personal by decreasing the need for additional stains.
The pathologists at the UMC Utrecht (The Netherlands) are using the Visiopharm Lymphnode Metastasis Detection App in a fully automated workflow:
- Reduced slide review time by over 35%
(3:41 min vs. 6:04 min per case) - Cut unnecessary IHC use, saving up to €3,000 over 16 weeks
- Maintained or improved sensitivity, especially for micro metastases
The result: faster workflows, fewer IHC stains, and no compromise on diagnostic safety.
Pathologists even had more fun evaluating the slides using AI.
Time Back in Your Day
More confidence in scoring can decrease your reviewing time, allowing you to spend more time on other cases. Reducing reflex testing allows you to sign off cases sooner, for the benefit of the patient and your workload.
Free up your lab resources
Experienced lab personal is rare. Make their life easier by reducing reflex testing for HER2 or IHC stains for lymph node metastasis, freeing up valuable resources in your lab for other tasks.
Cut costs
Save extra stains while keeping or even increasing sensitivity of the analysis. Save not only money through time, but also by reducing expensive secondary stains.

“Where we have seen the most significant impact of diagnostic digital breast pathology is in conjunction with HER2 assessment. The algorithm is simply better at discriminating between amplified and non-amplified cases, which means that we are seeing fewer inconclusive (2+) cases requiring additional (reflex) testing which is HER2-FISH testing, here at Vejle. This typically takes at least an additional day, and cost around EUR 250 per test (incl manual labor).”
Jan Lindebjerg, Vejle Hospital, Denmark
Precision pathology that works
We know, that implementing AI into digital pathology and into your existing workflow seems like an uphill task. Any disruption needs to be minimized, as AI is supposed to not only increase consistency of biomarker scoring, but also save you time. Thus, we are prioritizing integrations in many IMS/PACS and a zero-click workflow, minimizing manual interactions.
Ready for your review
Fully automated zero-click APPs – Results are ready when you are. No manual interaction needed, the whole slide is evaluated for scoring.
Integrations, that actually work
Designed for pathologists, loved by administrators. Easy for IT (web, on-prem or hybrid) as standalone through access to a slide scanner or compatible with existing digital IMS/PACS workflows.
Integration with Proscia, Sectra, PathAI, Philips, Smart in Media, Fujifilm, Corista, Paige, and more.
A decade of digital pathology expertise to serve you
No worries, we know setting things up can be tiresome and technical. That’s why we have a great team working on supporting you, knowing that every installation is different. Since our first IVD APP was released in 2014, we have gathered experience all across Europe in getting our customers up and running. Not as pilots – as integrated part of their workflow.
You’ll never walk alone
Our experienced support team gets you up and running quickly, and supports you along the way. These technical experts have worked with many other hospitals and labs and have a track record of just make things work.
Pioneering Since 2014 — Leading today
Quality matters. Our first IVD certified APP was released in 2014. Today, we are the only company with a full portfolio of clinical APPs certified under IVDR – and have been ahead of the field since 2022.

Scan with confidence
In Europe we offer various in vitro diagnostic use slide scanners to meet your slide throughput. Implement Visiopharm Scanners by Hamamatsu to get fast, reliable whole slide imaging, ready for image analysis.