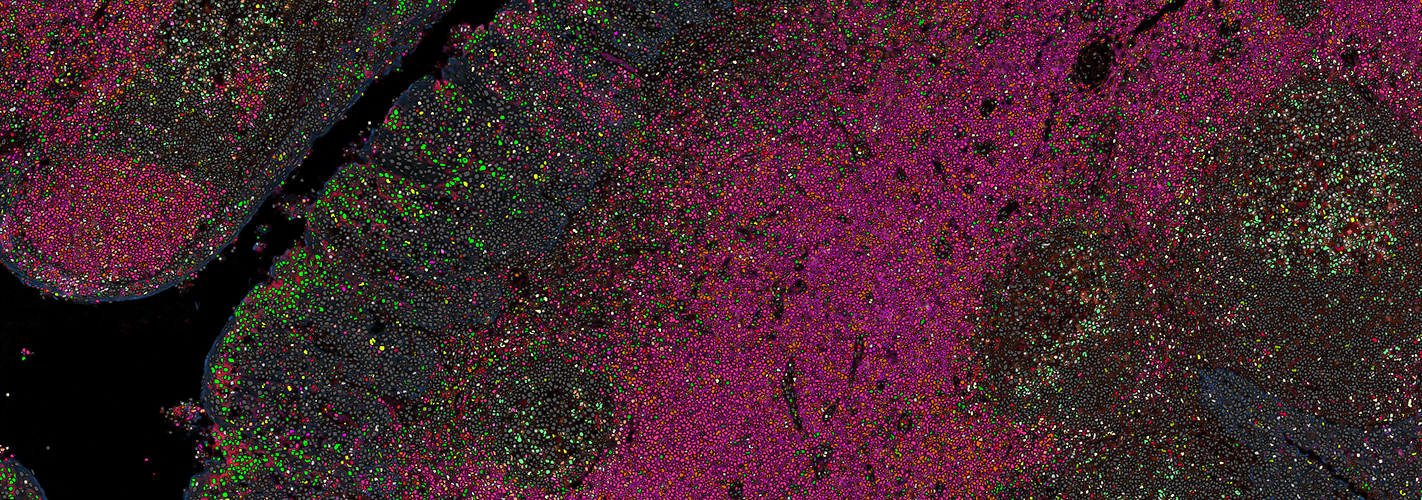
Visiopharm, a leader in AI-based digital pathology, and Ultivue, a leader in multiplexing for tissue biomarker analysis, today announced a new co-marketing partnership to promote a workflow with Ultivue´s UltiMapper I/O Immuno8 panel and subsequent image analysis. The workflow will standardize tissue biomarkers investigation of cellular immunophenotypes.
Visiopharm and Ultivue will deliver advanced tissue biomarker staining and analysis research solutions for immuno-oncology programs. The partnership leverages the companies’ capabilities and experience in multiplex immuno-profiling to enable its biopharma customers to carry out advanced analysis of the tumor microenvironment. The technologies from both companies are ideally suited to elevate biomarker discovery and analysis within the tumor microenvironment. For biomarker programs, the solution will provide actionable insights to advance tissue biomarker strategies.
Mark Rees, Ph.D., VP of corporate development, Ultivue, said:
“Ultivue have the best-in-class multiplex IF technology platform which together with our rapid custom service can deliver high-quality complex assay solutions to Biopharma and CRO partners in record turnaround times. Visiopharm offers the best image analysis technology for immunoprofiling; this exciting new partnership strengthens the workflow and analytics for advanced analysis of the tumor microenvironment, which ensures that we can deliver the essential data that is needed to expedite decision-making in biomarker programs.”
Adrian Arechiga, Ph.D., chief marketing officer, Visiopharm, said:
“Success in immunotherapy is driving the need for prognostic and predictive assays to inform patient selection and stratification. This requirement can be met by a combination of multiplexed staining, image analysis and artificial intelligence. This co-marketing partnership is underpinned by a joint vision of providing biopharma with exceptional tools for immunoprofiling through spatial analysis of tumor-immune cell interactions. Ultivue’s multiplexing kits and Visiopharm’s AI-based tissue mining tools are ideally suited to provide high-quality and in-depth insights that can move biomarker programs forward, ultimately resulting in the next generation of assays that may inform patient care.”
To learn more about this collaboration, products and events, see here.
 Visiopharm
Categories: Press Releases
13064
Visiopharm presents innovations for tissue biomarker standardization, and launches early access program at upcoming USCAP 2021 virtual meeting
Visiopharm
Categories: Press Releases
13064
Visiopharm presents innovations for tissue biomarker standardization, and launches early access program at upcoming USCAP 2021 virtual meeting
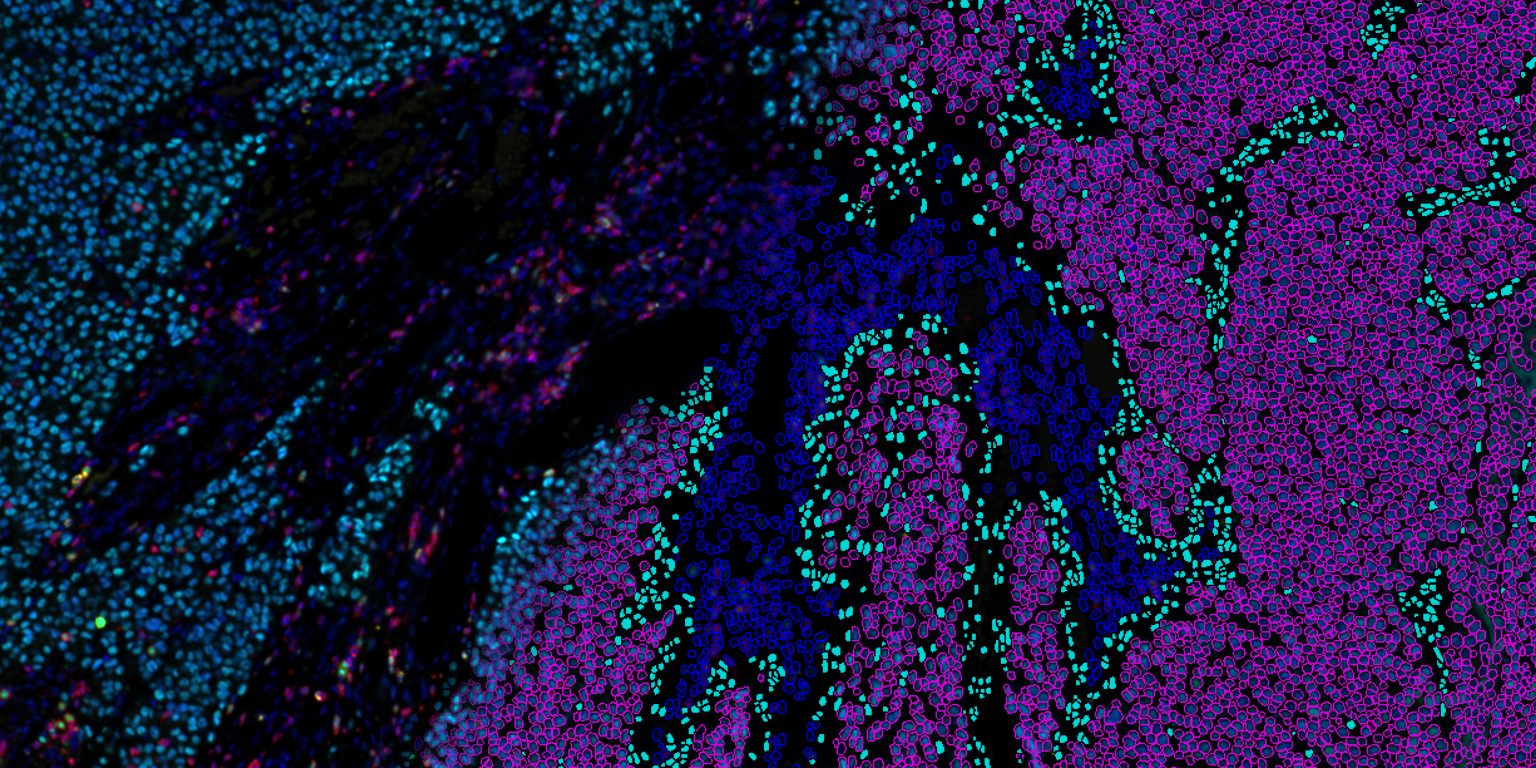
Visiopharm, a leader in AI-based digital pathology, announced it will be presenting innovations for tissue biomarker standardization at the upcoming 2021 United States and Canadian Academy of Pathology (USCAP) virtual meeting. In parallel, the company will launch its early access program to improve stain quality management, which is a critical aspect of standardized tissue biomarker assessment.
USCAP’s annual meeting is the largest pathology meeting in the US. The meeting consists of more than 40 divisions of specialized committee meetings, seminars, and presentations covering the latest innovations in pathology.
Visiopharm is enabling tissue pathology to enter the realm of precision medicine through a suite of AI-driven tools for precision pathology. AI is used for standardizing several steps along the journey from biopsy to data. Stain quality management and next generation image analysis are just two of the critical steps that will be discussed at USCAP.
At the Visiopharm sponsored seminar, the company will review the types and magnitude of standardization challenges in clinical workflows and offer potential solutions in precision pathology using digital image analysis based on AI. The seminar will furthermore discuss the regulatory pathways for digital and precision pathology.
“Visiopharm has over 20 years of experience and has mapped, quantified and mitigated error sources all along the value chain. We are taking radical and innovative approaches towards the standardized assessment of biomarkers. We look forward to discuss our observations and learnings with pathologists and other experts in the field at the upcoming seminar.”
Michael Grunkin, CEO, Visiopharm
Michael Grunkin, CEO, Visiopharm, said: “Visiopharm has over 20 years of experience and has mapped, quantified and mitigated error sources all along the value chain. We are taking radical and innovative approaches towards the standardized assessment of biomarkers. We look forward to discuss our observations and learnings with pathologists and other experts in the field at the upcoming seminar.”
Visiopharm will also unveil its Qualitopix early access program, providing selected participants access to their latest solutions to standardize tissue biomarker staining. The labs that join the program will be at the forefront of biomarker quality & standardization.
Dirk Vossen, chief diagnostic officer, Visiopharm, said: “AI-powered quantitative assessment of biomarker expression offers a route to improve the histological and immunohistochemical staining quality and interpretation of the expression of critical biomarkers such as PD-L1 and Ki67. We are excited to announce our early access program that provides quality and standardization solutions.”
To learn more about the early access program, click here.
 Visiopharm
Categories: Press Releases
12295
The importance of stain quality
Visiopharm
Categories: Press Releases
12295
The importance of stain quality
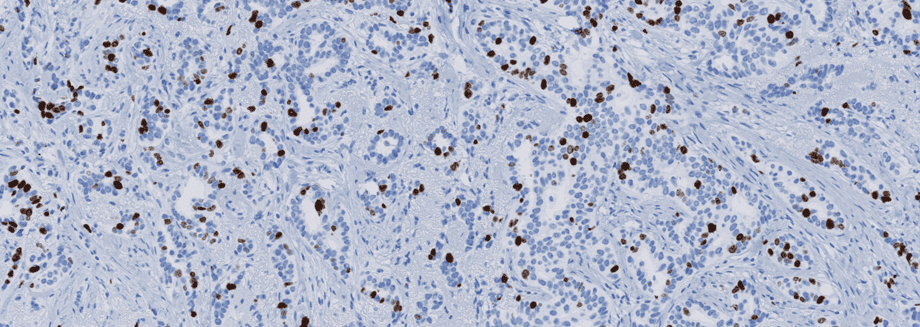
Immunohistochemical staining of tissue slides is a powerful tool that can provide pathologists and clinicians great insight for diagnosis, prognosis, and treatment of many disease states, particularly cancer.
However, like most tools that offer such significant benefits, it doesn’t come without challenges. The quality of staining in immunohistochemistry is an essential factor that may limit its effectiveness as a clinical tool. To correct the quality issues, it is vital to first understand how quality issues arise.
Stain quality can be described in terms of a signal-to-noise ratio (SNR), where signal refers to the level of expected epitope staining, and noise is the background component that results from cross-reactions with undesired epitopes. Insufficient SNRs are commonly the result of using poor or nonrobust antibodies and improper calibrations, the result of which mostly are false negative tests. Careful consideration for proper controls and adherence to quality control within the lab performing the staining protocol must be a priority to achieve optimal stain quality.1
An underlying reason for the variable quality being delivered by labs is the inherent complexity of an immunostaining protocol. Not only are protocols constantly being optimized, but with over 50 steps, each with multiple options, the staining protocol can have millions of variations. This has made standardization difficult.
One study conducted by the Nordic Immunohistochemical Quality Control (NordiQC) found an error rate in staining of almost 30% in labs participating in their study.
One study conducted by the Nordic Immunohistochemical Quality Control (NordiQC) found an error rate in staining of almost 30% in labs participating in their study.2 Dr. Vyberg, an expert in immunohistochemical quality control, noted that one reason for this high error rate is the use of in-house or “home-brew” kits rather than ready-to-use kits, which has direct consequences for treatments. Labs avoiding ready-to-use kits to save money on the front end could actually end up costing hospitals more in the long run.
An example is in the detection of the HER2 protein in breast cancer, one study calculated that every $1 labs saved by using in-house kits for HER2 rather than ready-to-use kits cost the hospital $6.3 However, quality assessment organizations have no authority to enforce underperforming labs to change their protocols.
This is not to suggest that all ready-to-use products are error-proof; internal standardization within each operating lab is critical with the use of any kit. Additionally, the field must be aware of the constantly evolving methods of staining in order to adopt the best possible protocols.
Early detection for some cancers is critical; delay in diagnosis and treatment increases the death rate considerably.4 Immunohistochemistry has the potential to help in early cancer detection, but first, the issue of stain quality must be addressed. One effort that is currently being made by NordiQC is the transparent sharing of optimal protocols for widespread use on their website. However, awareness and additional efforts are still needed, as solutions to this problem could directly impact cancer patients across the world.
1. Vyberg, M. & Nielsen, S. Proficiency testing in immunohistochemistry—experiences from Nordic Immunohistochemical Quality Control (NordiQC). Virchows Arch. 468, 19–29 (2016).
2. Nielsen, S. External quality assessment for immunohistochemistry: Experiences from NordiQC. Biotech. Histochem. 90, 331–340 (2015).
3. Vyberg M, Nielsen S, Røge R, Sheppard B, Ranger-Moore J, Walk E, Gartemann J, Rohr UP, Teichgräber V. Immunohistochemical expression of HER2 in breast cancer: socioeconomic impact of inaccurate tests. BMC Health Serv Res. 2015 Aug 29;15:352. doi: 10.1186/s12913-015-1018-6.
4. Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-JonescE, O’Sullivan DE, Booth CM, Sullivan R, Aggarwal A. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020 Nov 4;371:m4087. doi: 10.1136/bmj.m4087.
 Visiopharm
Categories: Blog Tags: quality
12469
Visiopharm appoints James Mansfield as senior vice president, research business development
Visiopharm
Categories: Blog Tags: quality
12469
Visiopharm appoints James Mansfield as senior vice president, research business development
Visiopharm is pleased to announce the appointment of James Mansfield as senior vice president, research business development.
James has worked in the sector for over 20 years and brings a wealth of experience, particularly internationally, in leading collaboration with scientists and partners. He also has a strong scientific publication record.
Most recently, James was Scientific Director at Magnetic Insight Inc, a start-up commercializing a novel molecular imaging technology called magnetic particle imaging (MPI).
Prior to this role, he had senior positions at Cambridge Research & Instrumentation, Inc (CRi) and PerkinElmer. During his time at PerkinElmer, he played a leading role in the team that commercialized multispectral imaging for pathology.
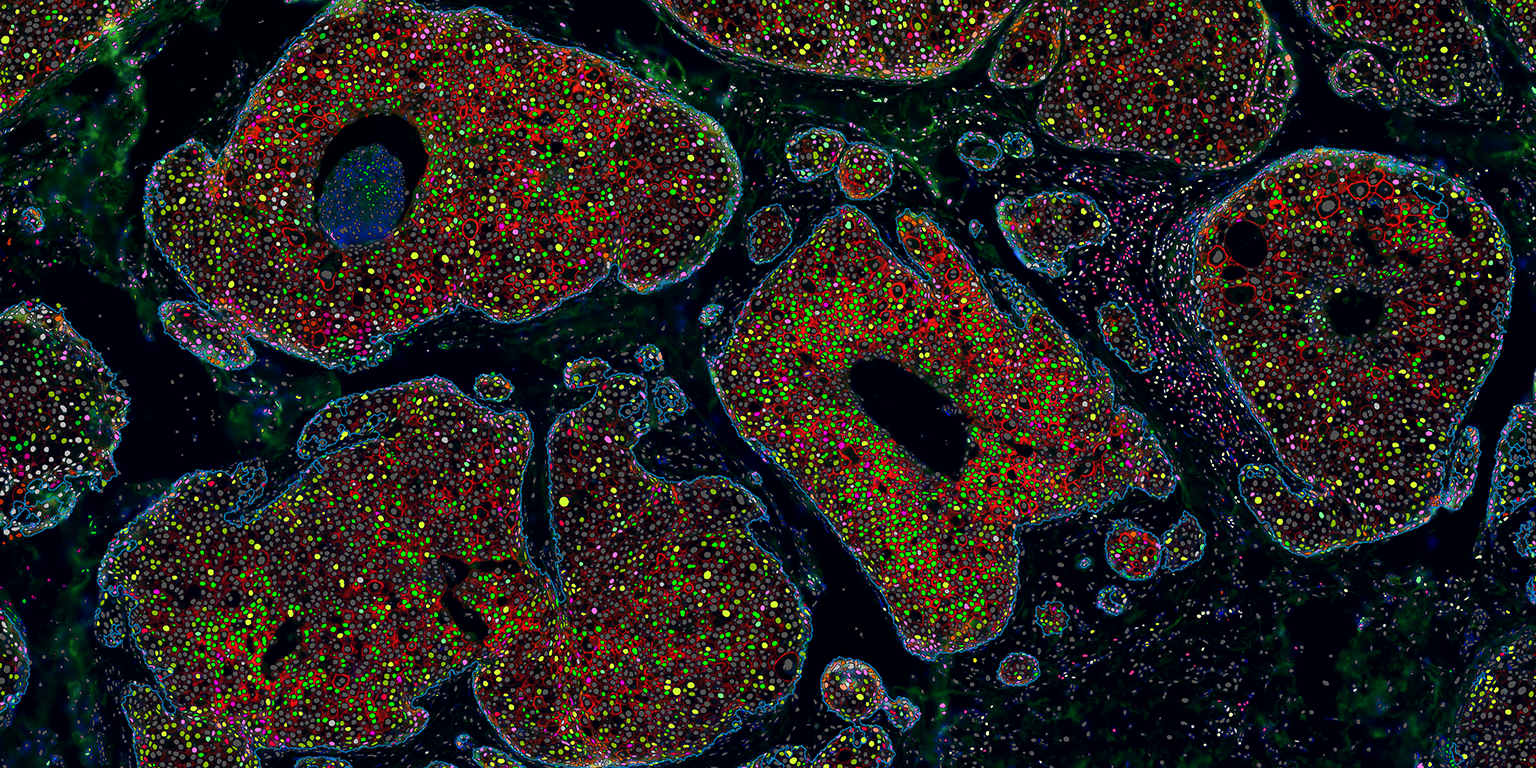
At Visiopharm, James will support the continued development of automated phenotyping software, support pharma services in team for biomarker discovery projects using multiplexed analysis, and advise our customer and clients on how to incorporate multiplexed analysis tools in their research programs.
In this role, James will also be driving our sales channel expansion strategies across Asia Pacific, where he has significant experience of introducing new imaging and analysis technologies and applications.
“The experience James brings in this highly specialized field, will not only allow us to continue to be the technology leaders in this field, but also support scientists is the most efficient use of such tools for their research.”
Michael Grunkin, CEO of Visiopharm
Michael Grunkin, CEO of Visiopharm said: “With the emergence of increasingly sophisticated multiplexed imaging techniques, tissue pathology offers an attractive spatially resolved and multi-dimensional platform for understanding the tumor-micro-environment. This in turn, is driving a demand for tools for analysis of increasingly complex image datasets, with uses in for example biomarker discovery projects. This demand is supported by our portfolio AI-driven automated phenotyping software tools.
“The experience James brings in this highly specialized field, will not only allow us to continue to be the technology leaders in this field, but also support scientists is the most efficient use of such tools for their research.”
James Manfield said: “Visiopharm has led the way in pathology analysis for two decades, and have always been the leader in multiplexed pathology analysis. The new emphasis on artificial intelligence for the segmentation of images into tissue types and for the multimarker phenotyping of immune and other cells is going to have a huge impact on cancer immunology research today, and eventually on clinical decision-making. I’m looking forward to working with the team on making the software even better for Visiopharm’s customers, and to helping grow multiplexed pathology analysis towards the clinic.”
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
