Proscia®, a leader in digital and computational pathology solutions, and Visiopharm, a world leader in AI-driven precision pathology software, today announced a strategic partnership to deliver integrated AI-enabled solutions that aim to improve clinical decision making for cancer care. Through the collaboration, the partners will advance the use of AI-enabled solutions to deliver new insights to pathologists for two of the most common cancer diagnoses.
Driven by a growing base of evidence on the economic and clinical benefits of adoption, leading laboratories are increasingly looking to adopt AI in routine practice to optimize operations and improve patient care. In a Nature survey of pathologists, 75% expressed interest in using AI to facilitate workflow and quality improvements, and 80% expected to be using AI by 2028. Powerful computational applications such as Visiopharm’s portfolio of breast and colon solutions have highlighted the role that AI could play in improving diagnosis for an increasingly large portion of lab volume. However, a key barrier to deriving the full value of AI is the lab’s ability to seamlessly integrate this diverse range of applications into routine operations.
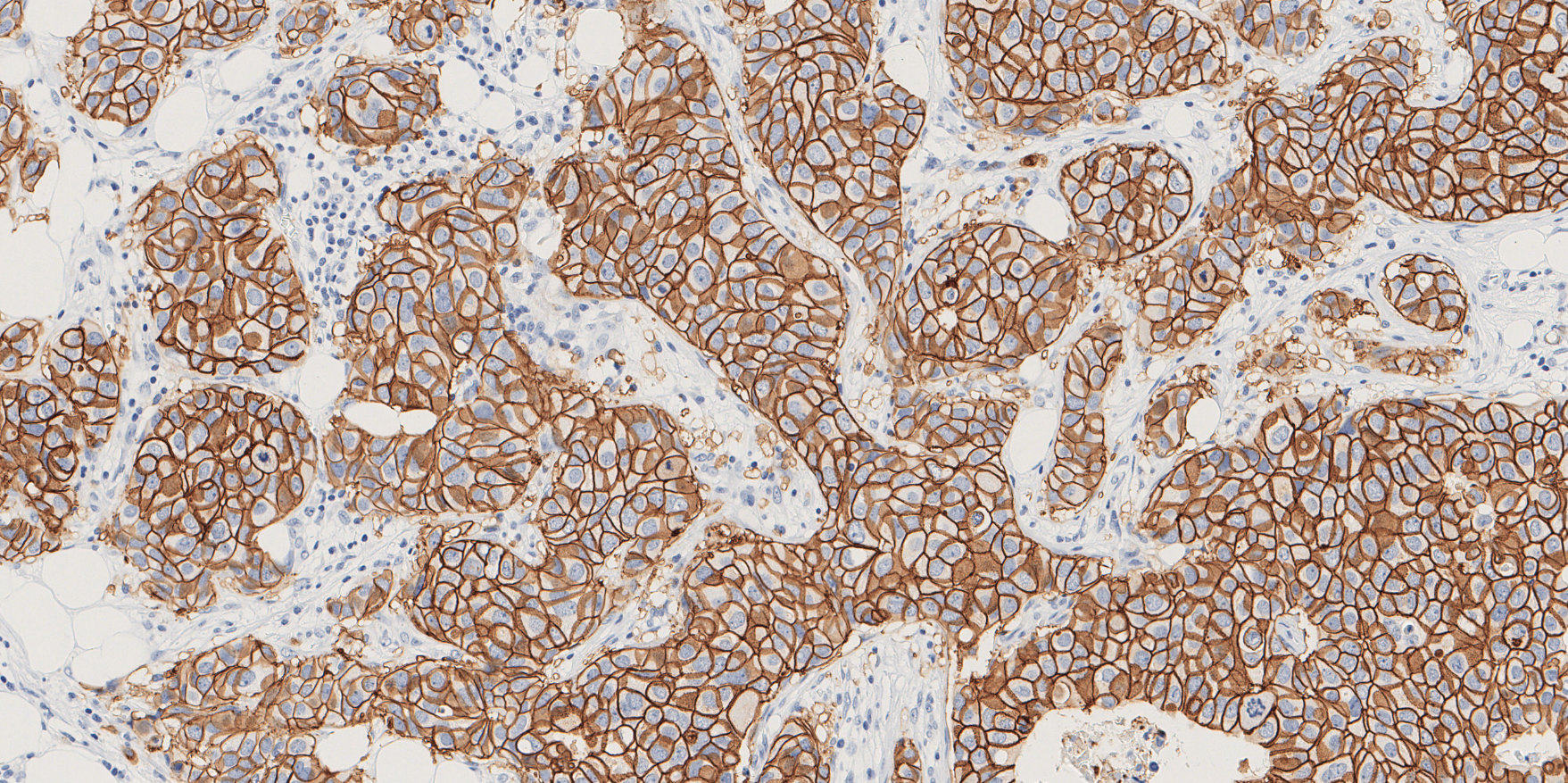
Proscia and Visiopharm have joined forces to deliver a unified solution that combines Visiopharm’s suite of CE-IVD* clinical AI applications – including breast IHC markers, breast metastasis detection, and colon metastasis detection – with Proscia’s CE-IVD Concentriq Dx platform** for image-based data and workflow management. Users of Concentriq Dx can now leverage the full suite of Visiopharm’s AI applications directly in the routine workflow, helping pathologists quantify the expression of biomarkers aiming to accelerate treatment decisions for patient care. This marks an expansion of the companies’ existing collaboration integrating AI-enabled solutions which have successfully driven breakthroughs in translational research and advance precision medicine for life sciences companies.
“We are excited about expanding our successful partnership in the research market to include the clinical market,” said Michael Grunkin, CEO of Visiopharm. “With Visiopharm’s commitment to AI-driven Precision Pathology, and Proscia’s commitment to interoperability and digital workflow solutions for pathology labs, this integrated solution combines efficient workflows and support for optimal treatment decisions to our clinical customers.” *
“Our unified solution will equip pathologists with powerful AI applications to quantify expression of biomarkers aiming to significantly improve treatment decisions,” said David West, CEO of Proscia. “Ultimately, this will enable pathologists to fully realize AI’s potential in unlocking insights that remain hidden to the human eye, helping to advance the way we understand and treat disease, driving accuracy in diagnosis, and establishing prognosis and personalized therapies for patients.”
Click here to learn more about how Visiopharm and Proscia are helping organizations to realize the full promise of their digital pathology data.
References
*Visiopharm’s clinical AI IVDs are certified under IVDR in Europe only. These are not for diagnostic purposes in US.
**Concentriq Dx is CE-marked for in-vitro diagnostic use in Europe and available for primary diagnosis in the US during the COVID-19 public health emergency.
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs support primary diagnostics. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 900 licenses and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, Netherlands and United States.
About Proscia
Proscia is a software company that is accelerating pathology’s digital transformation to change the way we understand diseases like cancer. Its Concentriq digital pathology platform and powerful AI applications are advancing the 150-year-old standard of research and diagnosis towards a data-driven discipline, unlocking new insights that accelerate discovery, improve patient outcomes, and fulfill the promise of precision care. Leading diagnostic laboratories and over 10 of the top 20 pharmaceutical companies rely on Proscia’s software each day. For more information, visit proscia.com.
 Visiopharm
Categories: Press Releases
17369
Visiopharm announces Oncotopix® Discovery with easy-to-use deep learning
Visiopharm
Categories: Press Releases
17369
Visiopharm announces Oncotopix® Discovery with easy-to-use deep learning
Visiopharm, a world leader in AI-driven precision pathology software, today announced Oncotopix® Discovery, a solution for tissue-based cancer research. This makes the most recent advances in AI and deep learning available as part of a comprehensive research platform.
Oncotopix Discovery is designed for all researchers and allows users to tackle both simple and the most complex datasets, to generate reliable quantitative results needed for breakthrough discoveries and publications.
Oncotopix Discovery provides:
Deep learning for all
Easy to use AI deep learning is now integrated as standard in all analysis packages for all tissue research applications.
See it, Train it, Find it
Anyone with an understanding of tissue morphology can train an AI deep learning APP to get accurate and reproducible results.
Pre-trained knowledge
New analysis packages include powerful pre-trained nuclei segmentation APPs suitable for brightfield and fluorescence applications which can be further tuned for even more specificity.
Best-in-class platform
Powered by Visiopharm’s best-in-class image analysis platform, combining 20 years of tissue expertise with innovative state-of-the-art technology.
Dirk Vossen, Visiopharm Chief Diagnostics Officer said:
“We’ve learned from our customers that although many recognise our software as best in class, particularly for demanding applications, there was an overall impression that the software was sometimes difficult to navigate and use. Therefore, it was best suited for use by experienced image analysis experts. We listened, and we have created Oncotopix Discovery, putting deep learning at the base of everything we provide. It means anyone, irrespective of their experience with image analysis software, can now get what they need from the best-in-class digital pathology image analysis solution.”
Louise Armstrong, Visiopharm Chief Commercial Officer said:
“Oncotopix Discovery enables users to get accurate and reliable results across a wide range of research applications including cancer research and neuroscience. Any user that understands cellular and tissue morphology can annotate slides to train our deep learning networks. By combining the researcher’s scientific knowledge with our tissue analysis expertise, anyone can generate the robust quantitative data needed for research studies and publication. We can’t wait to help a new group of biologists, medical researchers and pathologists accelerate their breakthroughs with Oncotopix Discovery.”
Learn more about Oncotopix Discovery.
About Visiopharm
Visiopharm® is a world leader in AI-driven precision pathology software. Their pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions, biopharmaceutical industry, and diagnostic centers. AI-based image analysis and tissue mining tools support research and drug development research worldwide, while CE-IVD APPs support primary diagnostics. With the most advanced and sophisticated artificial intelligence and deep learning, Visiopharm delivers tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2002 and is privately owned. The company operates internationally with over 900 licenses and countless users in more than 40 countries. The company headquarters are in Denmark’s Medicon Valley, with offices in Sweden, England, Germany, Netherlands and United States.
 Visiopharm
Categories: Press Releases
Visiopharm
Categories: Press Releases
