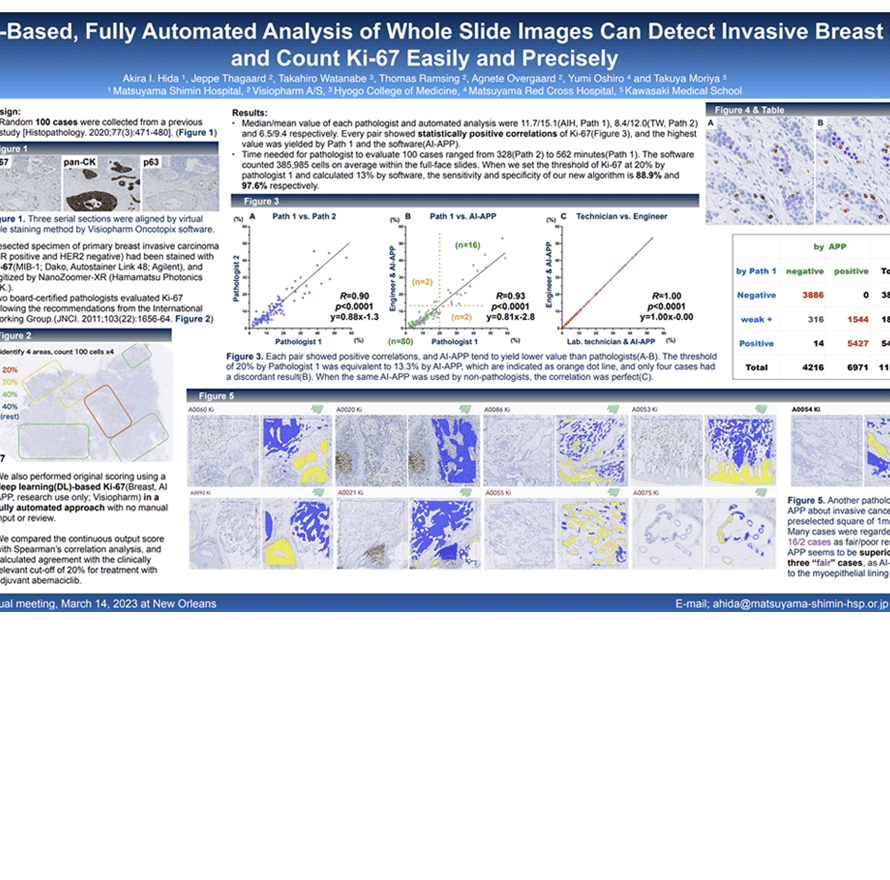

- The basic treatment of hormone-receptor(HR) positive breast carcinoma(BC) is antihormonal therapy.
- Recently, cyclin-dependent kinases 4/6 inhibitor called abemaciclib, has been approved for high risk patients after surgery, together with Ki-67 IHC MIB-1 pharmDx(Dako Omnis) for the patient selection.
- However, there remains concerns about reproducibility, and a development of automated image analysis is desired.
Akira I. Hida1, Jeppe Thagaard2, Takahiro Watanabe3, Thomas Ramsing2, Agnete Overgaard2, Yumi Oshiro4 and Takuya Moriya5
- Matsuyama Shimin Hospital
- Visiopharm A/S
- Hyogo College of Medicine
- Matsuyama Red Cross Hospital
- Kawasaki Medical School
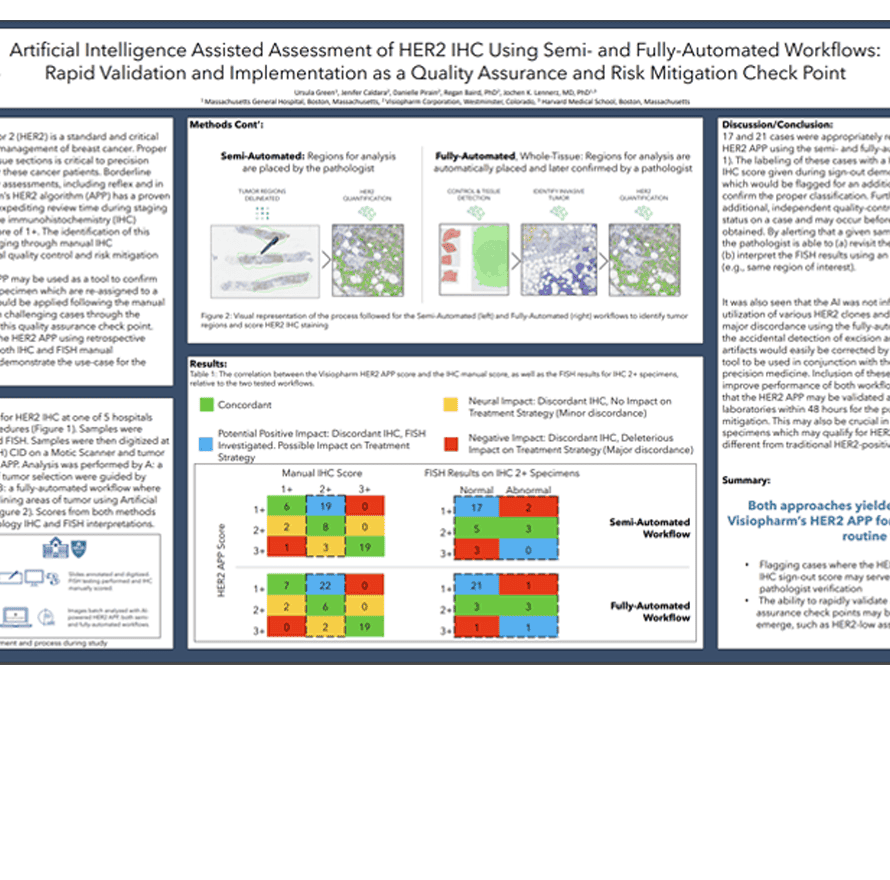

Human Epidermal Growth Factor Receptor 2 (HER2) is a standard and critical biomarker in the staging, diagnosis, and management of breast cancer. Proper classification of HER2 status in stained tissue sections is critical to precision pathology and personalized medicine for these cancer patients. Borderline cases require additional timely and costly assessments, including reflex and in situ hybridization (ISH) testing. Visiopharm’s HER2 algorithm (APP) has a proven track-record of assisting pathologists by expediting review time during staging and identifying borderline cases which are immunohistochemistry (IHC) positive, ISH normal as having a HER2 score of 1+. The identification of this population has been traditionally challenging through manual IHC interpretation, and AI can provide a crucial quality control and risk mitigation
checkpoint.
In this study, we suggest that the HER2 APP may be used as a tool to confirm manual sign-out assessments, or to flag specimen which are re-assigned to a new classification by the APP. This APP would be applied following the manual sign-out as a method of mitigating risk on challenging cases through the labeling of non-concordant reads during this quality assurance check point. Here we additionally validate the use of the HER2 APP using retrospective analysis on known patient samples with both IHC and FISH manual
assessments across multiple hospitals to demonstrate the use-case for the Center of Integrated Diagnosis (CID).
Ursula Green1, Jenifer Caldara2, Danielle Pirain2, Regan Baird, PhD2, Jochen K. Lennerz, MD, PhD1,3
- Massachusetts General Hospital, Boston, Massachusetts
- Visiopharm Corporation, Westminster, Colorado
- Harvard Medical School, Boston, Massachusetts


Understanding the rate of tumor cell growth in breast cancer specimens may be indicative of disease aggressiveness, a tumor characteristic which can be used to make an informed treatment decision. The nuclear protein Ki-67 is increased in cells as they prepare to divide, or proliferate, and is therefore widely used as a proliferation marker for tumor progression. This degree of tumor cell proliferation, or the proliferative index, is commonly detailed in pathology reports shared with the patient care team.
In this study, we utilized the Ki-67 [K2] immunohistochemistry (IHC) assay to stain 10 breast cancer specimens. Stained slides were imaged using the AT2 scanner (Leica Biosystems, Buffalo Grove, IL) and analyzed using the Visiopharm Image Analysis platform. Previous efforts to assess Ki-67 positivity utilizing image analysis have relied on the use of a secondary stain or manual effort by the pathologist to exclude non-invasive tumor regions. These antiquated methods are costly to the lab as they require additional materials or valuable pathologist time. Our novel image analysis approach utilizes artificial intelligence (AI) to automatically denote non-invasive verses invasive tumor regions, which can then be used to quantify the Ki-67 proliferative index. This valuable tool will allow for greater accuracy, cost-savings, and time efficiency when analyzing breast cancer samples compared to traditional methods.
Bhavika Patel, PhD1, Stephanie Allen1, Sameer Talwalkar, MD1, Navi Mehra, PhD1, Jeppe Thagaard2, Thomas W. Ramsing2, Agnete Overgaard, PhD2, Jenifer Caldara2
- Lanterne Dx, Boulder CO.
- Visiopharm Corporation
Download our guide on AI-assisted metastasis detection in lymph nodes today. This guide will provide you with valuable information on our CE-IVD certified AI deep learning application for detecting metastases in H&E stained lymph nodes associated with breast and colorectal adenocarcinoma.
In this episode of the podcast, Dr Aleksandra Zuraw interviews Dr Ralf Huss about digital pathology in clinical practice. They discuss the benefits of going digital, how to start the digital pathology journey, the role of pathologists in digital transformation, and how digital pathology is important for evaluating complex biomarkers and advancing immuno-oncology programs.
Further reading
Implementing digital pathology into clinical practice is a significant task, discussed by Dr Ralf Huss, chairman of Visiopharm’s advisory board. He shares his insights from his diverse experience in academia, big pharma, small startups, and hospital practice. The key to a successful transition to digital pathology is to provide interoperable tools that can be integrated into a functional pathology workflow. Labs should have a functional IT infrastructure and lab information management system. The new digital tools must have an open interface and be interoperable with the existing systems. Although digitalization tools have been available for two decades, few institutions have fully adopted it due to the lack of interoperability. The transition will be gradual, with interoperability playing a crucial role. In the long run, advances in medicine will drive pathologists to adopt digital tools, but they must be user-friendly, robust, and standardized.
Let us know what you think of the episode and any topics you would like us to cover in the future by sending us an email at: podcasts@visiopharm.com or contacting us on social media.
In this episode, Dr Aleksandra Zuraw interviews Prof David Harrison, a pathologist at University of St Andrews & Visiopharm’s advisor, about why pathologists should embrace AI in their work. David explains his journey in digital pathology, his role at iCAIRD, the benefits of AI, and overcoming regulation and implementation obstacles.
- Let us know what you think of the episode and any topics you would like us to cover in the future by sending us an email at: podcasts@visiopharm.com