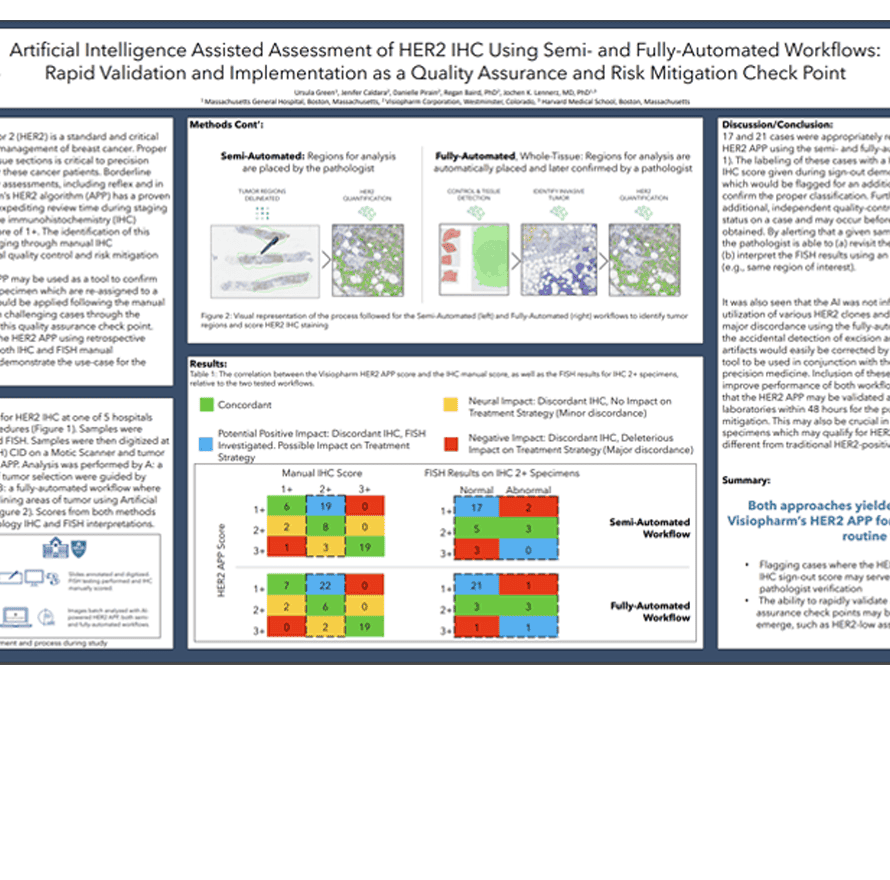

Human Epidermal Growth Factor Receptor 2 (HER2) is a standard and critical biomarker in the staging, diagnosis, and management of breast cancer. Proper classification of HER2 status in stained tissue sections is critical to precision pathology and personalized medicine for these cancer patients. Borderline cases require additional timely and costly assessments, including reflex and in situ hybridization (ISH) testing. Visiopharm’s HER2 algorithm (APP) has a proven track-record of assisting pathologists by expediting review time during staging and identifying borderline cases which are immunohistochemistry (IHC) positive, ISH normal as having a HER2 score of 1+. The identification of this population has been traditionally challenging through manual IHC interpretation, and AI can provide a crucial quality control and risk mitigation
checkpoint.
In this study, we suggest that the HER2 APP may be used as a tool to confirm manual sign-out assessments, or to flag specimen which are re-assigned to a new classification by the APP. This APP would be applied following the manual sign-out as a method of mitigating risk on challenging cases through the labeling of non-concordant reads during this quality assurance check point. Here we additionally validate the use of the HER2 APP using retrospective analysis on known patient samples with both IHC and FISH manual
assessments across multiple hospitals to demonstrate the use-case for the Center of Integrated Diagnosis (CID).
Ursula Green1, Jenifer Caldara2, Danielle Pirain2, Regan Baird, PhD2, Jochen K. Lennerz, MD, PhD1,3
- Massachusetts General Hospital, Boston, Massachusetts
- Visiopharm Corporation, Westminster, Colorado
- Harvard Medical School, Boston, Massachusetts