Pathology labs are facing 10-30% error rates in IHC. To address this challenge, we developed Qualitopix as a tool to measure staining intensity and ensure staining consistency.
Watch this short video to see how Qualitopix works.
Challenges of IHC standardisation
In this webinar, we will discuss the key challenges of IHC standardisation and you will be introduced to Qualitopix, an innovative solution that can be coupled with UK NEQAS ICC & ISH’s rigorous four-monthly assessments, enabling end-to-end quality assurance monitoring.
Learning objectives
- Understand the key challenges around analytic standardisation in immunohistochemistry (IHC), along with their root causes.
- Learn about Qualitopix, a practical and novel QA/QC solution for measuring analytical performance daily (including staining consistency) in IHC.
- Explore real-world examples and use cases of Qualitopix implementation in laboratories.
- Gain insights into regulatory changes and how Qualitopix can help meet these requirements.

Mateusz Tylicki, Product Manager at Visiopharm
Mateusz Tylicki is a dedicated AI Product Leader with a focus on Life Sciences and Healthcare. He is currently a Product Manager at Visiopharm, where he leads the development and validation of Qualitopix. In this role, Mateusz heads a multidisciplinary team committed to enhancing quality control and assurance in labs through AI technology. With over a decade of experience, Mateusz has held key product management roles at companies like Kheiron Medical and Antidote.me. His work has consistently centered on leveraging technology to improve patient care. He also served as a Product Management TA at General Assembly, helping to educate and mentor aspiring product managers. He holds a degree in Biological Science with Honors in Pharmacology from The University of Edinburgh and a Product Management certification from General Assembly. Based in Amsterdam, Mateusz is passionate about using AI to advance healthcare and improve lab operations.

Colin Tristram, CEO at Histocyte
Colin has spent the last twenty years developing, marketing, and commercializing a range of products for the IVD market, specifically tissue diagnostics. Colin is the founder and current CEO of HistoCyte laboratories. He studied Medical Microbiology at Newcastle University, specializing in immunology. His MSc focused on HER2 in breast cancer, involving the development of procedures for IHC controls. With this knowledge he and his colleagues started HistoCyte and developed a range of high-quality, reproducible, and cost-effective analyte control material for same-slide use in histopathology. HistoCyte was acquired by Atlas Antibodies in 2021.
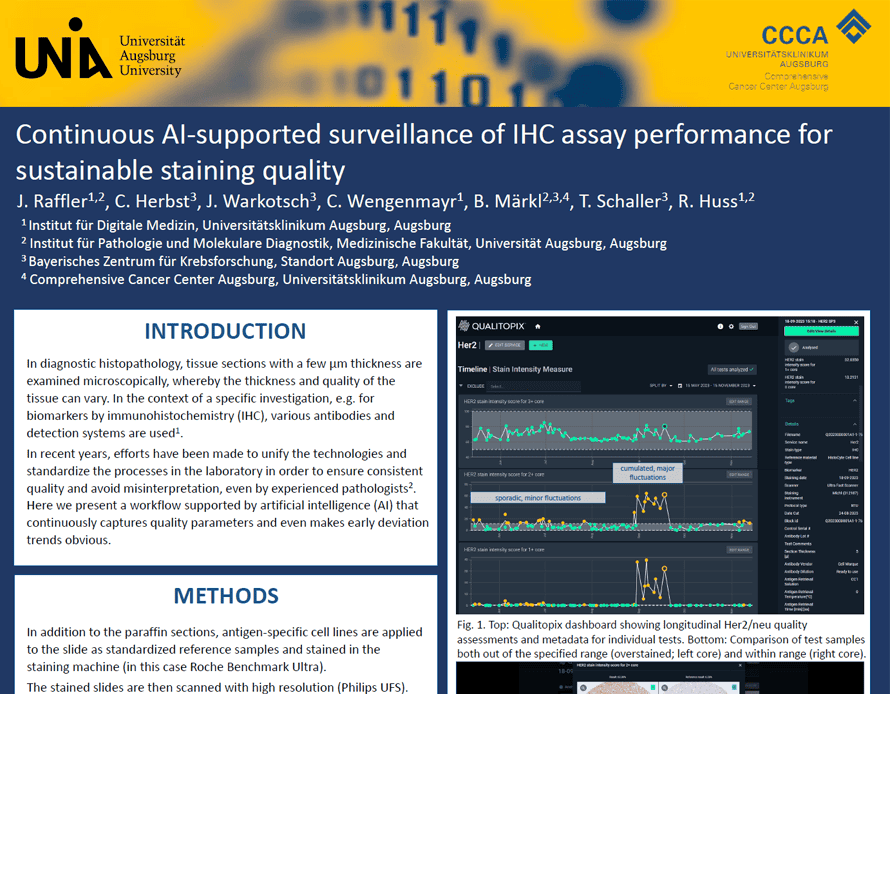
With discussions ongoing, that predictive IHC assays in the diagnostic lab need more standardization, the team from the Pathology Department of the University Hospital in Augsburg implemented a workflow using Qualitopix to continuously monitor staining parameter, capturing early deviations to allow immediate action.
J. Raffler1,2, C. Herbst3, J. Warkotsch3, C. Wengenmayr1, B. Märkl2,3,4, T. Schaller3, R. Huss1,2
- Institut für Digitale Medizin, Universitätsklinikum Augsburg, Augsburg
- Institut für Pathologie und Molekulare Diagnostik, Medizinische Fakultät, Universität Augsburg, Augsburg
- Bayerisches Zentrum für Krebsforschung, Standort Augsburg, Augsburg
- ComprehensiveCancer Center Augsburg, Universitätsklinikum Augsburg, Augsburg


Ki-67 is an important biomarker in breast cancer diagnosis, which gives prognostic information about the specific cancer. The resulting proliferation index of the tumor can support treatment selection and thus, correct assessment of Ki-67 levels is very important. To support the quality of the Ki-67 IHC staining in the respective labs, participation in external quality assessment (EQA) programs, like the ones offered by the UK National External Quality Assessment Scheme for Immunocytochemistry and In-Situ Hybridisation (UK NEQAS ICC & ISH) is recommended and has shown to improve laboratory performance.
In this study, UKNEQAS shows the application of new cell line based control materials to robustly identify false positive and suboptimal Ki-67 IHC staining using digital image analysis based on Visiopharm.
Fitim Berisha1, Dawn Wilkinson1, Lila Zabaglo1, Suzanne Parry1, Andrew Dodson1,2
- UK NEQAS ICC & ISH, 5 Coldbath Square, London EC1R 5HL UK
- Corresponding author: adodson@ukneqasiccish.org


Human epidermal growth factor receptor 2 (HER 2) is an important immunohistochemical (IHC) biomarker for breast cancer (BC) to determine the eligibility of HER 2 targeted therapies both for classical HER 2 overexpression and HER 2 low status For correct treatment decision, accurate and precise HER 2 IHC testing is fundamental. In this study, we evaluated if cell lines with relevant and critical expression levels of HER 2 in combination with artificial intelligence (AI) could be used to identify inaccurate HER 2 IHC assays.
Birgit Truumees1, Jeppe Thagaard2, Stine Amtoft Nielsen2, Mateusz Tylicki2, Lise Emanuelsen1, Søren Nielsen1
- NordiQC, Department of Pathology, Aalborg University Hospital, Denmark
- Visiopharm A/S, Denmark
Disclosures:
Birgit Truumees: None; Jeppe Thagaard: Employee, Visiopharm A/S; Stine Amtoft Nielsen: Employee, Visiopharm A/S; Mateusz Tyli ck i: Employee, Visiopharm A/S; Lise Emanuelsen: None; Søren Nielsen: None.
The Future of IHC: Opportunities and Challenges
In his lecture, Dr Huss will summarize the previous highlights of this extraordinary Masterclass from the need for quality control in precision pathology to the implementation of an automated and AI-based solution in a routine pathology workflow. With a special emphasis on the value of AI in controlling the robust performance of standardized IHC assays, Dr. Huss will advocate the importance to IHC tests to be acknowledged as an assay without increasing the burden on routine histopathology laboratories. On the contrary, such an approach could even compensate or at least forecast assay failure which allows early countermeasures from pre-analytical to post-analytical errors.

Prof Ralf Huss, BioM Biotech Cluster Dev. GmbH & University Hospital Augsburg, Germany
Ralf Huss is a Professor of Pathology and currently the Managing Director and CEO of the Biotechnology Development Agency in Munich, Germany. Prior to this role, he was the founding director of the Institute for Digital Medicine at the University Hospital Augsburg, Germany. Dr. Huss is board-certified in anatomical, experimental, and molecular pathology, with over 30 years of experience in international academic institutions and the pharmaceutical industry with a focus on histopathology, immunology, cancer research, and digital medicine.