Visiopharm appoints James Mansfield as senior vice president, research business development
Visiopharm is pleased to announce the appointment of James Mansfield as senior vice president, research business development.
James has worked in the sector for over 20 years and brings a wealth of experience, particularly internationally, in leading collaboration with scientists and partners. He also has a strong scientific publication record.
Most recently, James was Scientific Director at Magnetic Insight Inc, a start-up commercializing a novel molecular imaging technology called magnetic particle imaging (MPI).
Prior to this role, he had senior positions at Cambridge Research & Instrumentation, Inc (CRi) and PerkinElmer. During his time at PerkinElmer, he played a leading role in the team that commercialized multispectral imaging for pathology.
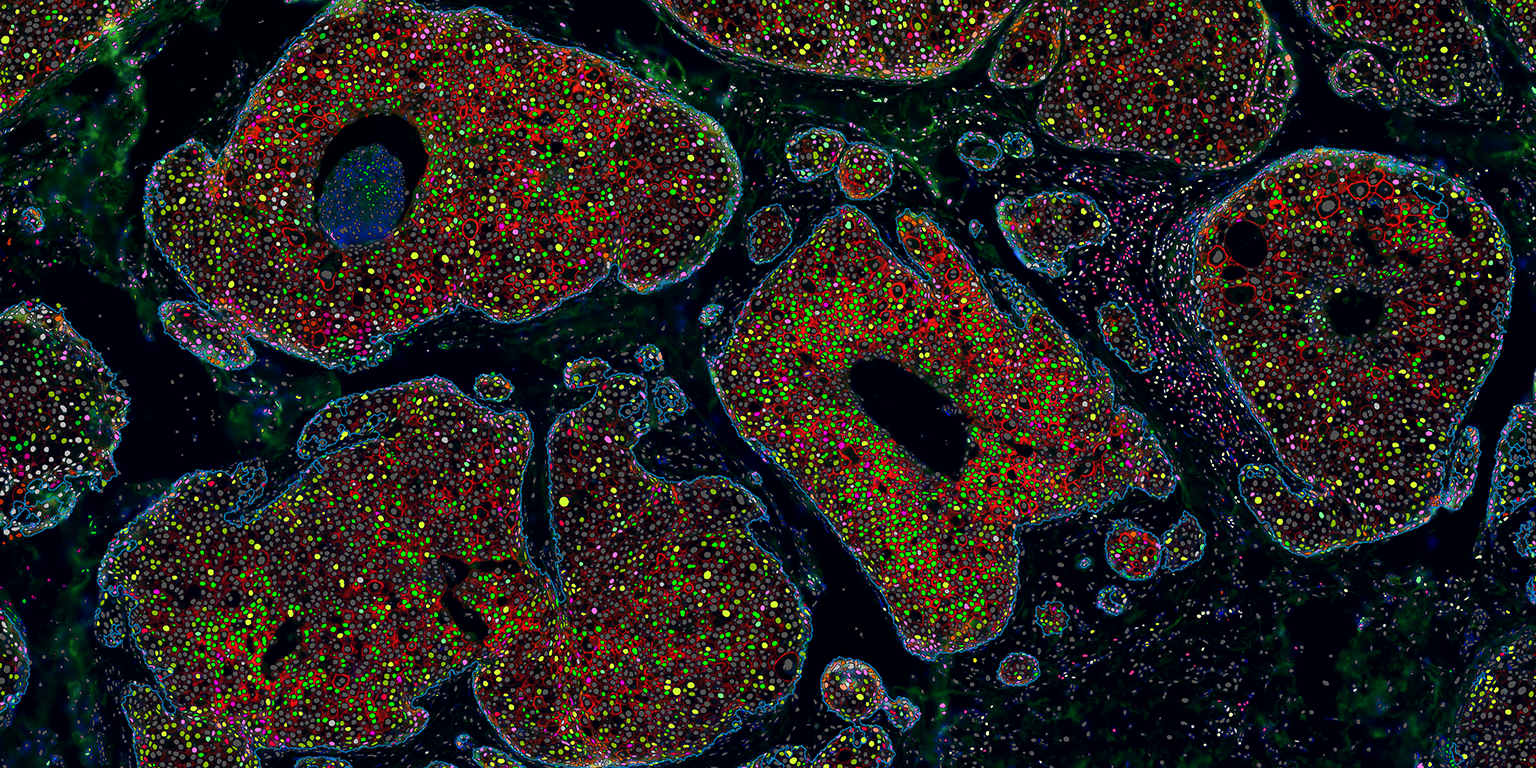
At Visiopharm, James will support the continued development of automated phenotyping software, support pharma services in team for biomarker discovery projects using multiplexed analysis, and advise our customer and clients on how to incorporate multiplexed analysis tools in their research programs.
In this role, James will also be driving our sales channel expansion strategies across Asia Pacific, where he has significant experience of introducing new imaging and analysis technologies and applications.
“The experience James brings in this highly specialized field, will not only allow us to continue to be the technology leaders in this field, but also support scientists is the most efficient use of such tools for their research.”
Michael Grunkin, CEO of Visiopharm
Michael Grunkin, CEO of Visiopharm said: “With the emergence of increasingly sophisticated multiplexed imaging techniques, tissue pathology offers an attractive spatially resolved and multi-dimensional platform for understanding the tumor-micro-environment. This in turn, is driving a demand for tools for analysis of increasingly complex image datasets, with uses in for example biomarker discovery projects. This demand is supported by our portfolio AI-driven automated phenotyping software tools.
“The experience James brings in this highly specialized field, will not only allow us to continue to be the technology leaders in this field, but also support scientists is the most efficient use of such tools for their research.”
James Manfield said: “Visiopharm has led the way in pathology analysis for two decades, and have always been the leader in multiplexed pathology analysis. The new emphasis on artificial intelligence for the segmentation of images into tissue types and for the multimarker phenotyping of immune and other cells is going to have a huge impact on cancer immunology research today, and eventually on clinical decision-making. I’m looking forward to working with the team on making the software even better for Visiopharm’s customers, and to helping grow multiplexed pathology analysis towards the clinic.”
Visiopharm® is a world leader in AI-driven Digital Pathology solutions. Visiopharm’s pioneering image analysis tools support thousands of scientists, pathologists, and image analysis experts in academic institutions and the biopharmaceutical industry.
AI-based image analysis and tissue mining tools support research and drug development research worldwide, while their CE-IVD APPs support primary diagnostics. With the most advanced and sophisticated artificial intelligence and deep learning, they deliver tissue data mining tools, precision results, and workflows.
Visiopharm was founded in 2001 and is privately owned. The company operates internationally with over 900 licenses and countless users in more than 40 countries. Company headquarters are in Denmark’s Medicon Valley, with further offices in Sweden, United Kingdom, Germany, and the United States. Follow Visiopharm on Linkedin and Twitter. For other news, visit the Visiopharm Newsroom.

