The advent of targeted therapies using the HER2 receptor as a delivery mechanism in breast cancer, introduced a need for re-evaluation HER2 scoring in previously classified negative cases (2+ ISH -ve; and 1+), as patients with these tumours may benefit from these innovative treatments. At The Christie Hospital. Manchester, U.K., our specialised unit (BTRU) has been exclusively reporting ER, PR, and HER2 status in breast cancers for the past 25 years with a dedicated staff, adhering to consistent technical guidelines and stringent UKAS accreditation standards, with an annual assessment of approximately 1,600 cases. This study aimed to evaluate the performance of an AI solution in the identification of HER2 ultra-low compared to a cohort of reported HER1+ and 0 cases that were manually scored by the BTRU.
Xu Xuan Lim, MBChB1, Angela Cramer, CLS1, Jeppe Thagaard, PhD2, Thomas Wichmand Ramsing2, Pedro Oliveira, MD1
- The Christie NHS Foundation Trust
- Visiopharm
Manual biomarker scoring can be both time-consuming and inconsistent — challenges that become even more critical with classifications like HER2-low and -ultralow. The Insight platform enables AI-driven, fully automated image analysis APPs to provide precise, reliable biomarker scoring. With Insight, you can eliminate variability and focus on what truly matters: making confident, informed decisions.
DeepDx Prostate (RUO), developed by Deep Bio, is an AI-powered research tool for quantitative prostate biopsy analysis. It provides structured, scalable, and automated insights, enabling data-driven research with precision and confidence. Designed to enhance efficiency, it streamlines and optimizes laboratory workflows.
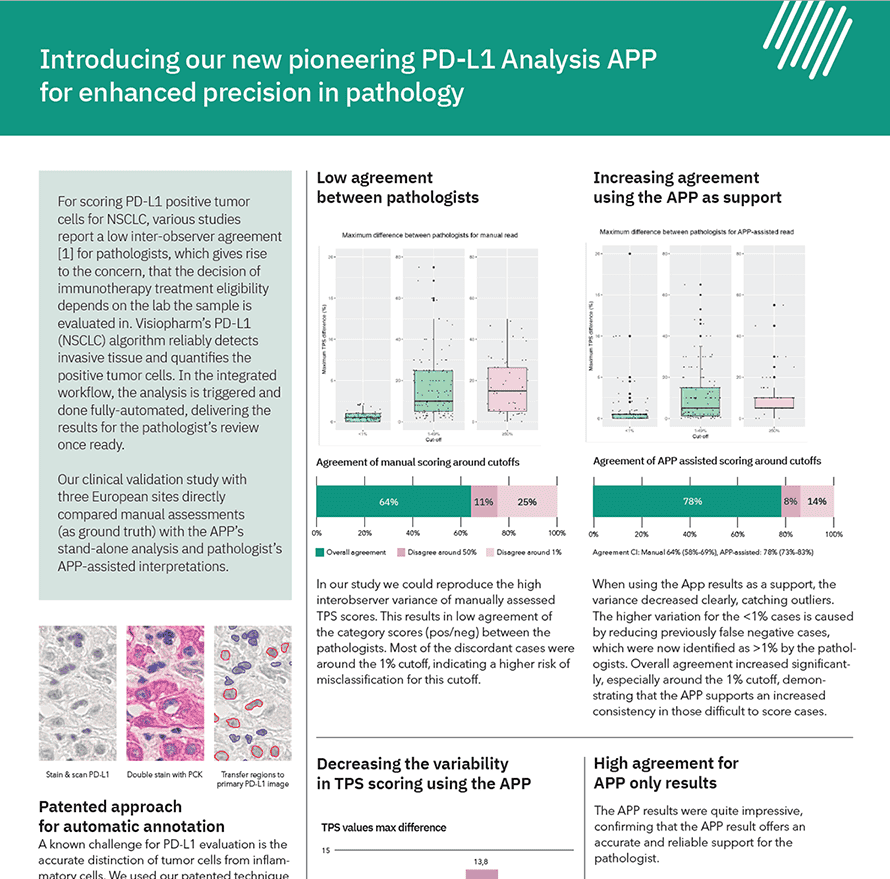
For scoring PD-L1 positive tumor cells for NSCLC, various studies report a low inter-observer agreement [1] for pathologists, which gives rise to the concern, that the decision of immunotherapy treatment eligibility depends on the lab the sample is evaluated in. Visiopharm’s PD-L1 (NSCLC) algorithm reliably detects invasive tissue and quantifies the positive tumor cells. In the integrated workflow, the analysis is triggered and done fully-automated, delivering the results for the pathologist’s review once ready.
Our clinical validation study with three European sites directly compared manual assessments (as ground truth) with the APP’s stand-alone analysis and pathologist’s APP-assisted interpretations.
[1] Troncone G, Gridelli C; doi: 10.21037/tlcr.2017.10.05
Progress with certainty in your tissue biomarker analysis.
Insight Entreprise workflow – Zero click automated analysis.
Automatically initiated based on information (e.g. a tissue and stain type) from PACS/IMS.
Progress with certainty in your tissue biomarker analysis.
Insight Entreprise workflow – Zero click automated analysis.
Automatically initiated based on information (e.g. a tissue and stain type) from PACS/IMS.