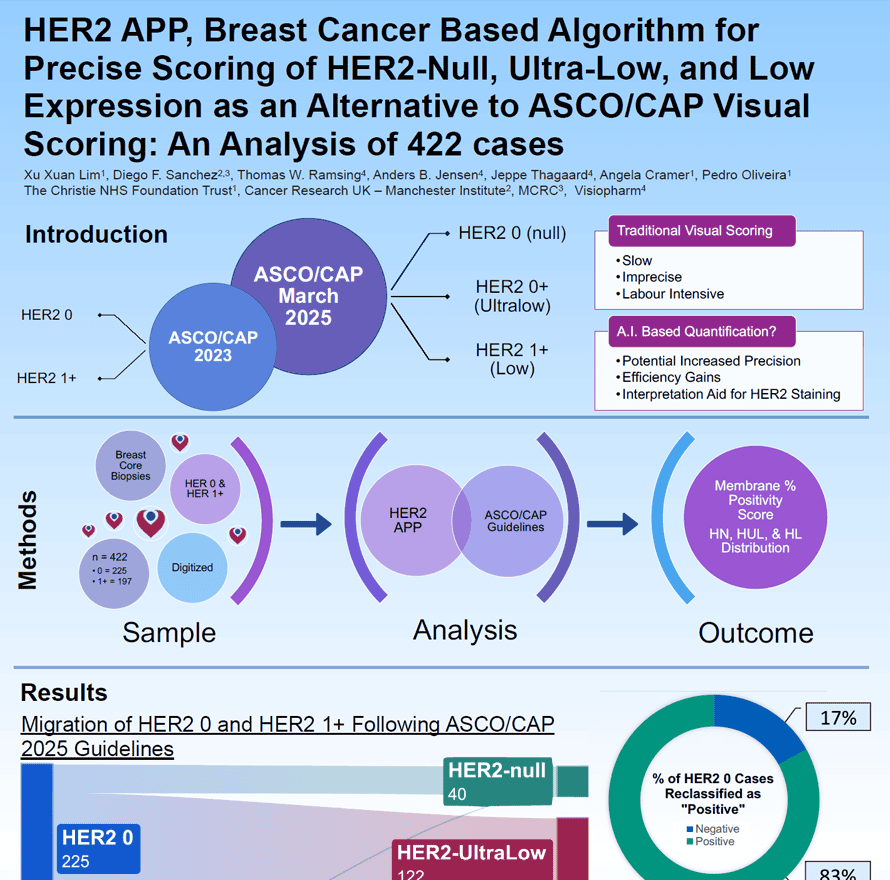

Accurate HER2 assessment in breast cancer is essential for guiding targeted therapy, yet traditional visual scoring is challenging. The updated ASCO/CAP reporting template adds HER2-null, ultra-low, and low categories, increasing interpretation complexity. This study evaluates an AI-based algorithm (HER2 APP) for precise, efficient quantification of HER2 membrane positivity in 422 digitized biopsies.
Xu Xuan Lim1, Diego F. Sanchez2,3, Thomas W. Ramsing4, Anders B. Jensen4, Jeppe Thagaard4, Angela Cramer1, Pedro Oliveira1
- The Christie NHS Foundation Trust
- Cancer Research UK – Manchester Institute
- MCRC
- Visiopharm
Manual biomarker scoring can be both time-consuming and inconsistent — challenges that become even more critical with classifications like HER2-low and -ultralow. The Insight platform enables AI-driven, fully automated image analysis APPs to provide precise, reliable biomarker scoring. With Insight, you can eliminate variability and focus on what truly matters: making confident, informed decisions.
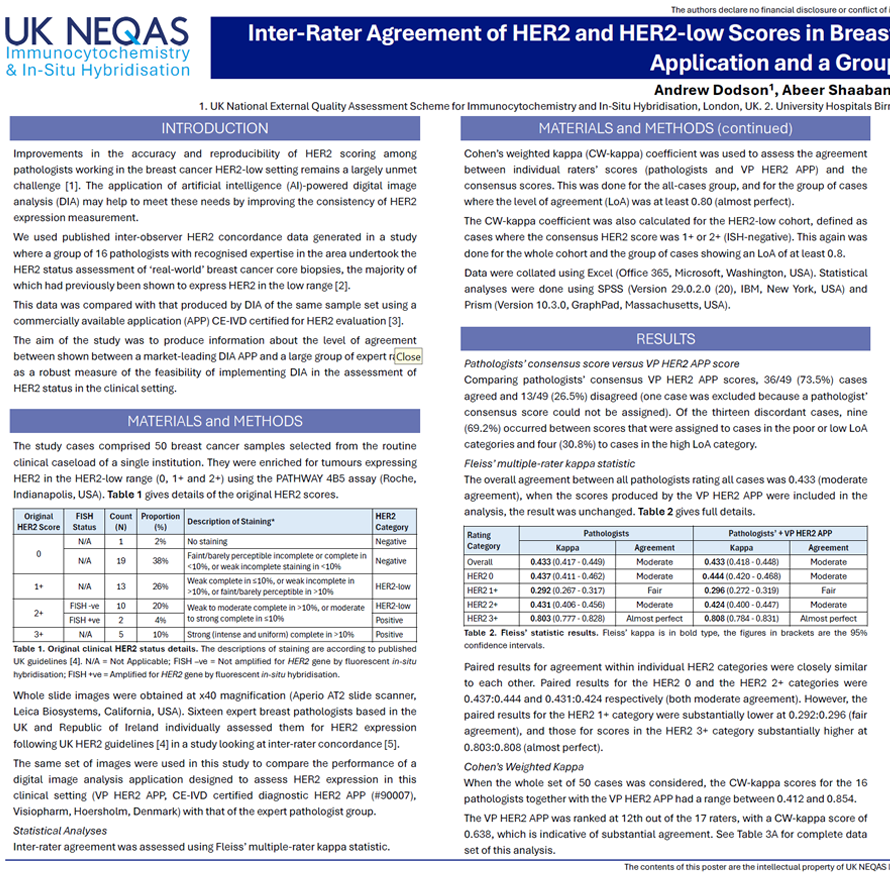

Improvements in the accuracy and reproducibility of HER2 scoring among pathologists working in the breast cancer HER2-low setting remains a largely unmet challenge. The application of artificial intelligence (AI)-powered digital image analysis (DIA) may help to meet these needs by improving the consistency of HER2 expression measurement.We used published inter-observer HER2 concordance data generated in a study where a group of 16 pathologists with recognised expertise in the area undertook the HER2 status assessment of ‘real-world’ breast cancer core biopsies, the majority of which had previously been shown to express HER2 in the low range.This data was compared with that produced by DIA of the same sample set using a commercially available application (APP) for HER2 evaluation. The aim of the study was to produce information about the level of agreement between shown between a market-leading DIA APP and a large group of expert raters as a robust measure of the feasibility of implementing DIA in the assessment of HER2 status in the clinical setting.
Andrew Dodson1, Abeer Shaaban2, Lila A Zabaglo1, Suzanne Parry1
- UK National External Quality Assessment Scheme for Immunocytochemistry and In-Situ Hybridisation, London UK.
- University Hospitals Birmingham NHS Foundation Trust Queen Elizabeth Hospital, Birmingham, UK.


- Accurate assessment of lower ranges of HER2 immunohistochemical (IHC) expression has become important since trastuzumab deruxtecan (T-DXd) was approved for treatment of patients with HER2-low metastatic breast cancer (BC).
- Findings from DESTINY-Breast06 Phase 3 trial showed that T-DXd offers a favourable outcome over standard chemotherapy in patients with HER2-low and HER2-ultra-low metastatic BC.
- Several studies showed low concordance among pathologists in distinguishing low levels of HER2 IHC expression.
- There is an emerging need for a standardized and reproducible HER2-low testing methodology.
- A dedicated EQA programme for the assessment of IHC stain quality of HER2-low testing was established by UK NEQAS ICC & ISH in 2023 providing support to clinical laboratories and gathering evidence about the reproducibility of HER2-low testing.
- We report results from the application of digital image analysis (DIA) for the HER2-low IHC assessment with a view of implementing DIA in the HER2-low EQA programme.
Lila Zabaglo1, Suzanne Parry1, Dawn Wilkinson1, Andrew Dodson1
- UK National External Quality Assessment Scheme for Immunocytochemistry and In-Situ Hybridisation, London UK.
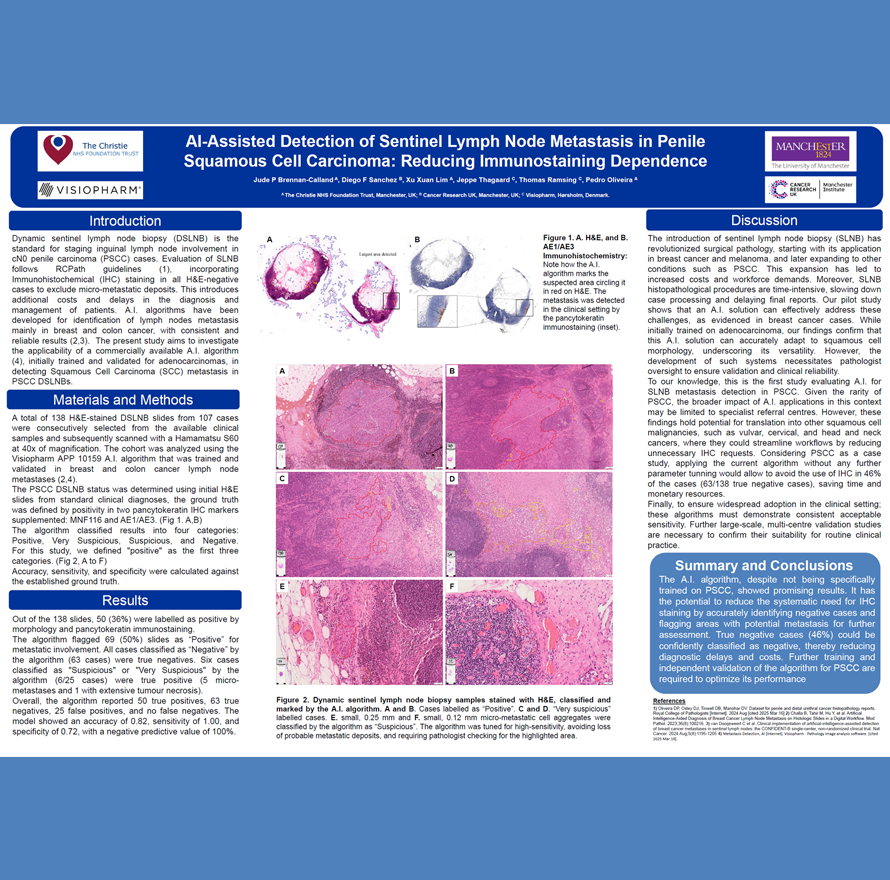

The growth in cancer immunotherapy agents requires an understanding of the immune contexture of the tumor microenvironment (TME). This can be aided by high-plex imaging and analysis to obtain phenotypes of specific cells and study their biodistribution and interactions. Imaging Mass Cytometry (IMC) is the method of choice for single-step staining and high-plex imaging of tissues, avoiding the complications of autofluorescence and cyclic imaging.
IMC has expanded its capabilities with three distinct imaging modes: Preview, Cell, and Tissue. The Preview Mode is a rapid scanning system that captures a comprehensive overview of the stained tissue, mapping out the distribution of over 40 markers and revealing tissue heterogeneity. This enables researchers to make informed decisions about which areas warrant closer examination on the same. Building on this, Cell Mode offers high-resolution imaging for detailed analysis of the Regions of Interest (ROIs) identified during Preview, all using the same slide. Tissue Mode complements these by providing a fast acquisition of the entire tissue at a lower resolution, which is optimal for quantitative pixel-based analysis of tissue biology. These modes support automated, continuous imaging of more than 40 large tissue samples (400 mm2) weekly. Following Preview Mode, the selection of ROIs for high-resolution imaging is a critical step, enhanced by automated AI algorithms to ensure it is informed by biomarker expression.
Jude P Brennan-CallandA, Diego F SanchezB, Xu Xuan LimA, Jeppe ThagaardC, Thomas RamsingC, Pedro OliveiraA
- The Christie NHS Foundation Trust, Manchester, UK
- Cancer Research UK, Manchester, UK
- Visiopharm, Hørsholm, DenmarkA/S
Recent advances in antibody-drug conjugates (ADCs) demand more nuanced classifications of the full spectrum of HER2. However, reproducibility of manual HER2-low and -ultralow scoring is challenged due to a scoring classification not feasible for the human eye. A better way to score HER2 is required.
Introducing our new, fully automated HER2 algorithm:
Enabling precise quantification of expression across the complete spectrum.