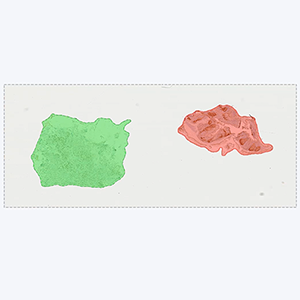
Tissue detection
Detection of sample tissue and exclusion of control tissues.
#90004
The Ki-67 protein is associated with cellular proliferation and can be assessed by Ki-67-immunohistochemical (IHC) staining. The calculated proliferation index can be correlated to the tumor grade and the clinical course.
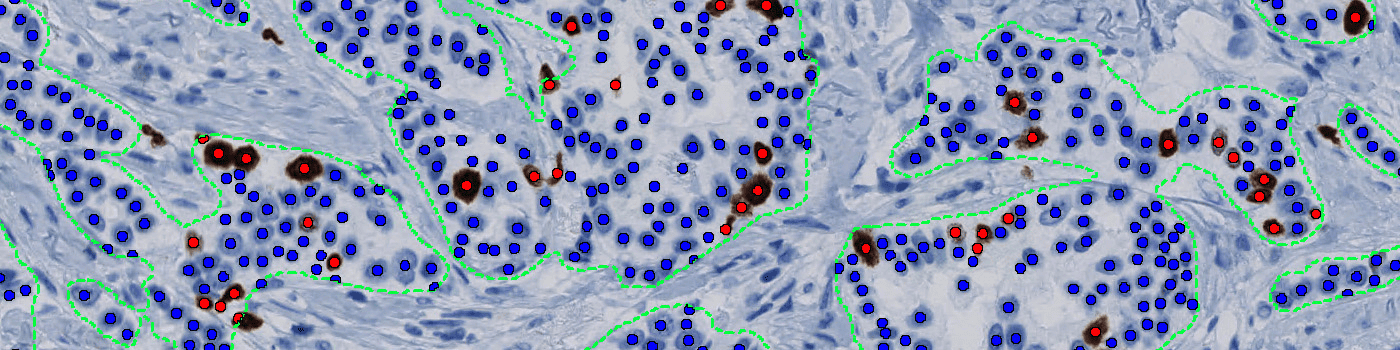
Our Ki-67 solution is fully automated and does not require manual input during analysis. The solution consists of four automated analysis steps. Once started, it automatically separates the sample tissue from control tissues and robustly identifies invasive cancer areas in the sample. Tumor nuclei are counted based on their Ki-67 expression and the resulting proliferation index for the whole tumor area is calculated.
In EU/UK: CE IVD – for use in diagnostic procedures
Quantitative Output variables
The output variables obtained from this protocol are:
Workflow
Step 1: After scanning, the PACS/LIS/IMS informs the Visiopharm software about the new image and the analysis of the image is automatically started.
Alternatively, the analysis can be started manually by opening the image and starting the analysis.
Step 2: Pathologist review the analyzed image. If needed, adjustments can be made. Once reviewed, pathologists sign off the case in the system.
Additional information
Compatibility
The solution works with stains from Agilent, Roche, and Leica. It can be applied to ductal or lobular breast cancer samples.