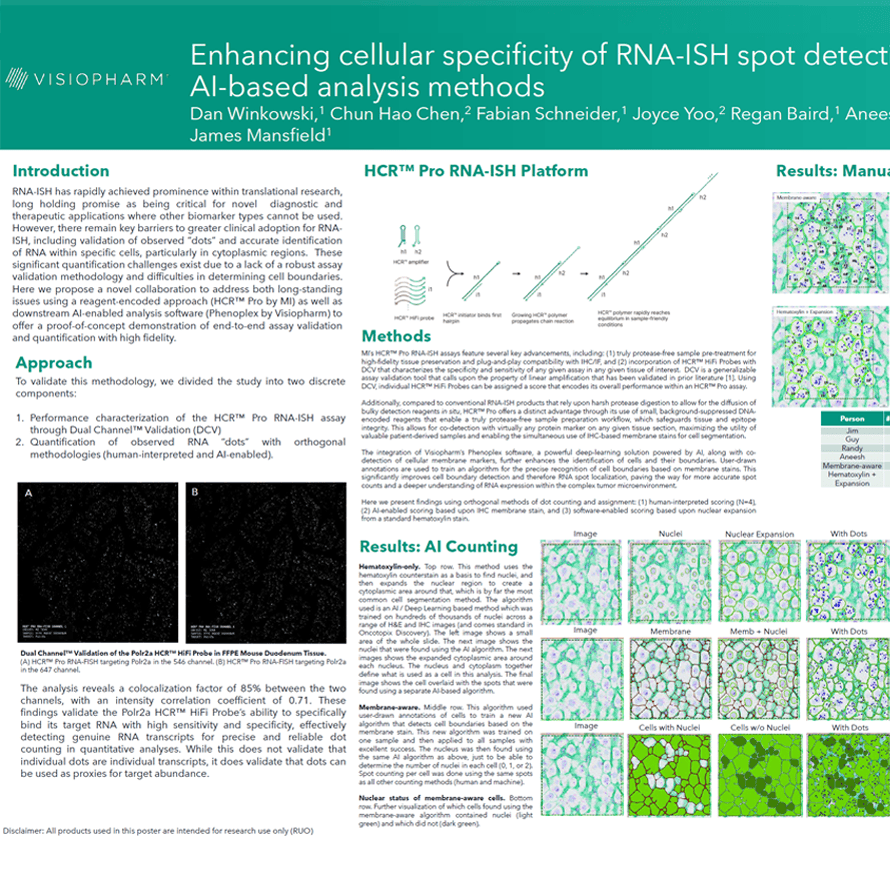
RNA-ISH has rapidly achieved prominence within translational research, long holding promise as being critical for novel diagnostic and therapeutic applications where other biomarker types cannot be used. However, there remain key barriers to greater clinical adoption for RNAISH, including validation of observed ”dots” and accurate identification of RNA within specific cells, particularly in cytoplasmic regions. These significant quantification challenges exist due to a lack of a robust assay validation methodology and difficulties in determining cell boundaries. Here we propose a novel collaboration to address both long-standing issues using a reagent-encoded approach (HCR™ Pro by MI) as well as downstream AI-enabled analysis software (Phenoplex by Visiopharm) to offer a proof-of-concept demonstration of end-to-end assay validation and quantification with high fidelity.
Dan Winkowski1, Chun Hao Chen2, Fabian Schneider1, Joyce Yoo2, Regan Baird1, James Mansfield1
- Visiopharm A/S, Horsholm, Denmark
- Molecular Instruments, Los Angeles, CA, USA