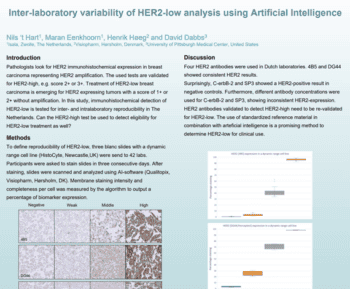
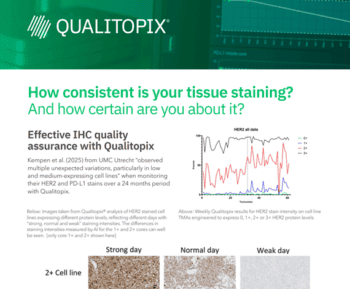
Tissue staining is a critical step in the diagnosis and prognosis of various diseases, especially cancer. However, tissue staining quality can vary significantly due to factors such as reagents, protocols, instruments, even operators. This can lead to inconsistent and inaccurate results, affecting patient care and clinical research.
In this seminar presented at PathVisions 2023, Martin will discuss challenges for managing stain quality of immunohistochemical (IHC) and H&E, potential root causes and a practical QA/QC solution. Qualitopix uses artificial intelligence (AI) to analyze standardized, validated test materials over time, complementing external quality assessment (EQA)-led proficiency testing. He will go through real-world examples and research conducted through the last 12-18 months.
- Importance of Staining Consistency: One of the main takeaways is the critical role that staining consistency plays in accurate diagnostics. Ensuring consistency is vital for reliable test results in immunohistochemistry.
- Role of Technology and AI: The seminar highlighted how advancements in AI and the development of new tools can significantly aid in monitoring and improving staining quality, thereby enhancing the overall diagnostic process.
- Need for Standardization and Quality Control: The talk emphasized the need for standardization in staining procedures and the use of quality control measures. This is essential to cope with the challenges posed by the complexity and variability of tests in pathology labs.

Martin Kristensson
Senior Vice President, Global Clinical Sales