Alimentiv’s digital pathology innovation with Visiopharm: an interview with Dr. Pavine Lefevre
Alimentiv is at the forefront of delivering digital pathology solutions through its combination of a state-of-the-art specialty histology laboratory, AcelaBio, and digital image analysis services, powered by Visiopharm® software. This end-to-end offering of high-quality sample processing and quantitative biomarker analysis provides our clients with robust insights into histopathology, drug mechanisms of action, target engagement, and pharmacodynamics.
In this recent interview, Alimentiv’s Lead Scientist, Pavine Lefevre PhD, shared her insights on Alimentiv’s histopathology services and the advantages of the Visiopharm® platform.
Dr. Pavine Lefevre is a distinguished scientist specializing in precision medicine and digital pathology. She currently serves as the Lead Scientist at Alimentiv, a contract research organization focused on gastrointestinal diseases. With over 10 years of experience collaborating with clinicians, scientists, and pharmaceutical organizations, Dr. Lefevre oversees translational research exploring histopathology, drug mechanisms of action, pharmacodynamics, and pharmacokinetics. She utilizes cutting-edge technologies in molecular and cellular biology, customizing approaches based on client needs to ensure the success of clinical trials and ultimately improve human health.

Visiopharm: Can you tell us about Alimentiv, your services, and how you differentiate yourself from other CROs?
Dr. Pavine Lefevre: Alimentiv is a specialized CRO driving innovation in clinical trials for inflammatory bowel disease (IBD) and other GI conditions, such as celiac disease and eosinophilic esophagitis. We provide comprehensive support for drug development, guided by leading gastroenterologists and scientists. Alimentiv delivers exceptional medical imaging expertise, supporting endoscopic and histopathological scoring for critical clinical trial data. In combination with our specialty CAP/CLIA-certified laboratory, AcelaBio, we offer seamless end-to-end workflows for GI tissue biopsy analysis. This includes spatial transcriptomics, multiplex immunofluorescence, digital pathology, and advanced image analysis. We not only support exploratory endpoints in clinical trials but also drive internal research to deepen our understanding of IBD histopathology, ultimately accelerating drug development and improving patient outcomes.
Visiopharm: What problems were you trying to solve when you started working with us compared with previous analysis software, and how did Visiopharm help you overcome those challenges?
Dr. Pavine Lefevre: Initially, we relied on external vendors for digital image analysis, a valuable step in our early development. However, to enhance our capabilities and streamline our workflow, particularly after establishing AcelaBio as our in-house histopathology laboratory offering advanced staining techniques like multiplex IHC/IF, we decided to bring digital image analysis in-house. Quantitative image analysis, under pathologist oversight, was crucial for achieving precise measurements and tackling complex research questions.
We chose Visiopharm for its comprehensive software that perfectly aligned with our business needs, complemented by their exceptional customer support and training. This strategic move empowered our precision medicine team to deliver more in-depth tissue characterization and advance our understanding of disease mechanisms.
Visiopharm: What specific features of our software do you find most helpful?
Dr. Pavine Lefevre: Visiopharm’s Author module allows us to develop highly customized, high-performance algorithms (“APPs”) by offering exceptional flexibility in parameter and setting selection. The introduction of the AI Author module, featuring deep learning-based classification, has changed our analysis capabilities, delivering robust and precise results.
We’ve achieved significant success using the TissuealignTM, TissuearrayTM, and PhenoplexTM modules in combination with the AI Author, finding these tools to be great to work with.
Visiopharm: Can you share a specific case study where Visiopharm’s software helped you solve a particularly challenging problem in your research or analysis?
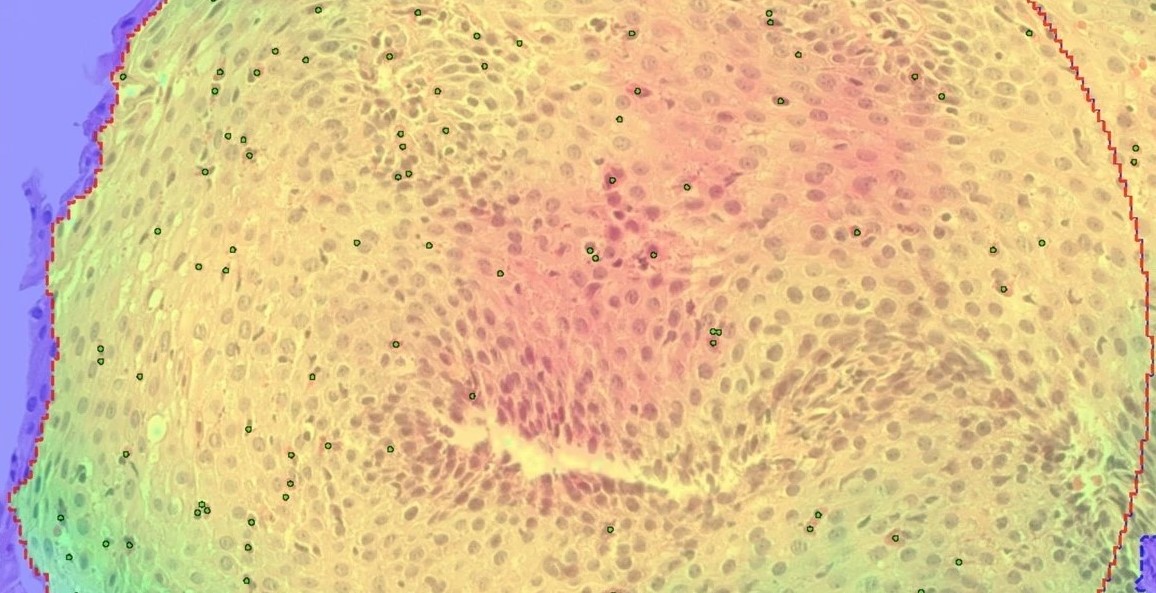
Dr. Pavine Lefevre: Last year at Digestive Disease Week (DDW), we presented our digital pathology analysis algorithm designed to automate peak eosinophil count (PEC) quantification in eosinophilic esophagitis biopsies (Figure 1). This project aimed to create a computer-aided tool to assist pathologists in accurately quantifying PEC from whole slide images of H&E-stained esophageal biopsies in clinical trials. We collaborated with Drs. Evan Dellon (UNC) and Arjan Bredenoord (Amsterdam UMC), who provided esophageal biopsy images for algorithm training and development. The images were manually annotated by pathologists and used to train and develop an eosinophil counting APP with Visiopharm.
The algorithm was developed and iteratively refined, through validation, to accurately: detect the tissue area, detect and count eosinophils across the entire tissue section, and precisely identify and count eosinophils within a 40x high-powered field hotspot, representing areas of peak eosinophil density. Validation via Sensitivity Assessment of the APP demonstrated a strong correlation between the APP’s automated PEC and pathologist manual counts (Spearman r = 0.9895). We aim to use this APP to facilitate augmented reading in the near future.

Visiopharm: Could you share some insights into how the software has added value to your business and your customers?
Dr. Pavine Lefevre: Acquiring Visiopharm software has enabled us to expand our business capabilities, allowing us to offer a seamless, end-to-end digital pathology solution from biopsy processing to sophisticated image analysis.
By leveraging Visiopharm, we achieve rapid, quantitative biomarker analysis with enhanced precision and efficiency. This translates to richer, more comprehensive insights into critical aspects of histopathology, drug target engagement, and pharmacodynamics.
Visit Alimentiv’s website to learn how our precision medicine services can advance your research. Discover our comprehensive services, advanced technology, and proven expertise.
Learn more about Visiopharm software here.
About Alimentiv, Inc.
Alimentiv is a leading specialty GI-focused CRO, advancing frontiers of gastrointestinal (GI) clinical trials and medical research since 1986. As a global CRO offering clinical, medical imaging and precision medicine services, Alimentiv partners with pharmaceutical and biotechnology industries to advance the development of novel therapies and accelerate their time to market. Alimentiv is headquartered in London, Ontario, Canada, with a global footprint across its operations in Canada, the United States, Europe, Asia-Pacific, and Latin America. For more information, visit www.alimentiv.com.
About Visiopharm
Visiopharm is a leading provider of AI-driven precision pathology software for research and diagnostics. In research, it is a technology leader providing tools that help scientists, pathologists, and image analysis experts produce accurate data for all types of tissue-based research. In diagnostics, it is a leader within clinical applications, with no fewer than nine diagnostic algorithms cleared under IVDR for EU and UK customers. These applications provide diagnostic decision support and can be easily activated and integrated into existing lab workflows. Founded in 2002, Visiopharm is privately owned and operates internationally with over 750 customer accounts in more than 40 countries. The company’s headquarters are located in Denmark’s Medicon Valley, with legal entities in Sweden, the UK, Germany, the Netherlands, and the United States, and local representation in France and China.



