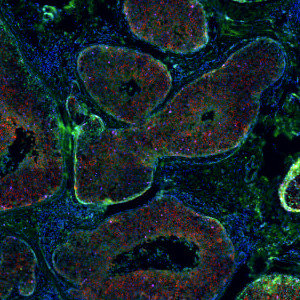
Breast cancer panel section of a tissue region.
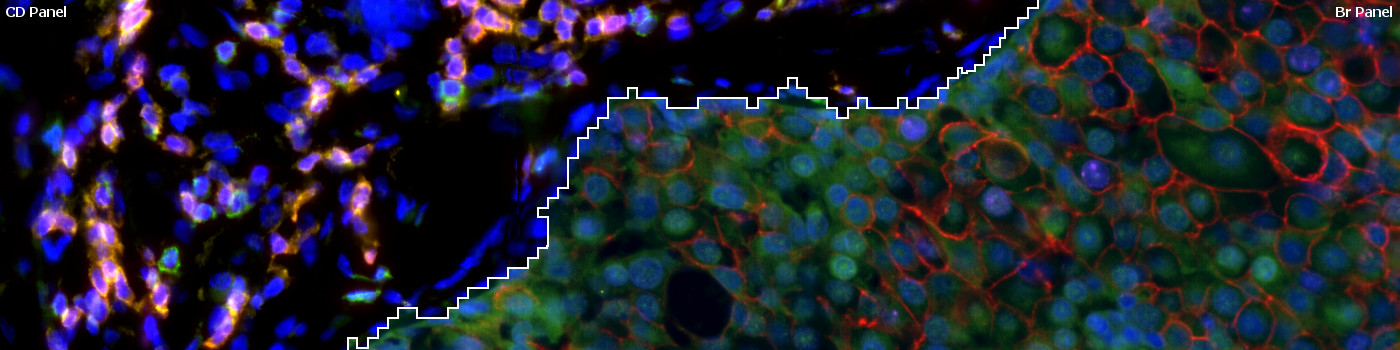
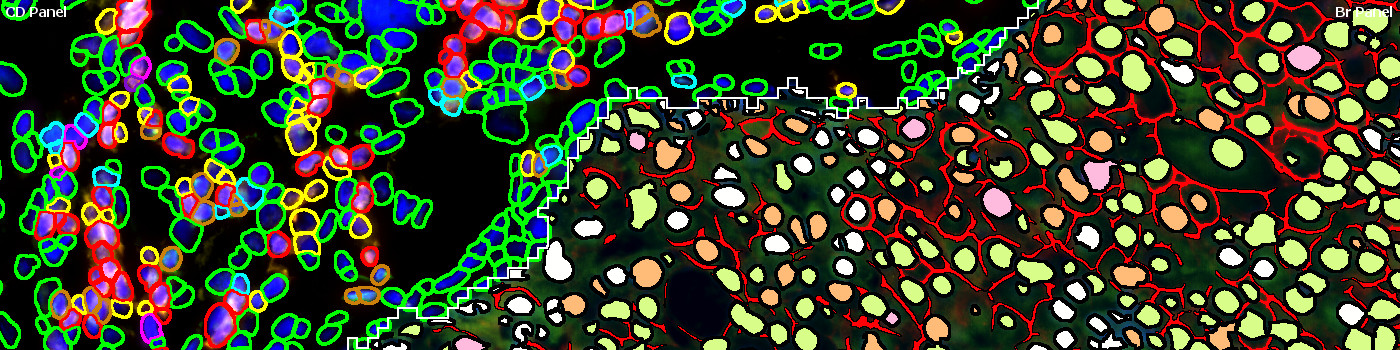
#10141
The widely used biomarkers for breast cancer: ER, PR, Ki-67, and HER2 are combined with four CD biomarkers (CD3, CD4, CD8, and CD20) in a virtual fluorescence 8-plex multiplex setting. Two serial sections are stained with the breast cancer biomarkers and CD biomarkers, respectively, and aligned using our patented Tissuealign™ module. While maintaining a 4-plex staining method, all 8 biomarkers are displayed simultaneously in the aligned image. Due to co-expressers, this assay yields a total of 12 different phenotypes that can all be detected with the “10141 – Virtual 8-plex Multiplexing, Breast Cancer, TME” APP.
Auxiliary APPs
APP: “01 Detect Tumor and Stroma”
The auxiliary is used for automatic tumor detection. Some manual editing of ROIs may be necessary.
Quantitative Output variables
The output variables obtained from this protocol are:
CD PANEL
Br PANEL
Workflow
Step 1: Load and run the APP “01 Detect Tumor and Stroma” for tumor and stroma identification.
Step 2: Load and run the APP “02 Classify Stroma_CD Nuclei” for classification of nuclei based on fluorophores within stromal regions.
Step 3: Load and run the APP “03 Classify Tumor_Br Nuclei” for classification of nuclei based on fluorophores within tumor regions.
Step 4: Load and run the APP “04 Classify HER2” for the detection of HER2 positive cells within tumor regions.
Methods
The breast cancer (Br) and CD panel sections are aligned using the Tissuealign™ module, with the Br panel as channel 1 and CD panel as channel 2. Next the tumor and stroma regions are automatically identified and the nuclei within each compartment are detected. The stroma nuclei are detected based on the dapi color and shape on the CD panel section, while tumor nuclei are detected based on the dapi color and shape on the Br panel section. The detected nuclei are then classified based on their fluorophore intensity on the CD panel section (stroma nuclei) or the BR panel section (tumor nuclei). Finally, the tumor regions of the Br panel section are analyzed for HER2 positivity.
Staining Protocol
Cell IDX Ultraplex Multiplex Technology, see [1]. Cell IDx has developed an expanding range of next-generation, modified haptens which are used to label primary antibodies. Primary antibodies are combined in cocktails of four antibodies and then detected with a panel of anti-hapten secondary antibodies each labeled with a different fluor. The result is a simple two-hour, two-step staining procedure yielding the type of data previously impossible.
Keywords
CD3, CD8, CD4, CD20, HER2, ER, PR, Ki67, multiplex, co-registration, align, co-localization, image analysis, tumor micro environment, fluorescence
References
USERS
This APP was developed for David Schwartz, CEO and founder of Cell IDx, a San Diego based company revolutionizing multiplexing staining and biomarker detection in intact tissue. Images were provided by Cell IDx.
LITERATURE
1. Cell IDx, San Diego, California. Introducing UltraPlex Multiplexing Technology. Accessed: August 23, 2019.