Accelerate insights in drug and biomarker discovery
Discovering new targets and predictive biomarkers for targeted treatments necessitates a deep understanding of the tissue micro-environment. To gain this accurate information, extensive multi-spectral/modal and complex image datasets need to be reliably analyzed to fully grasp cell interactions. To ensure accurate downstream decision-making, the analysis and result verification of these datasets are crucial.
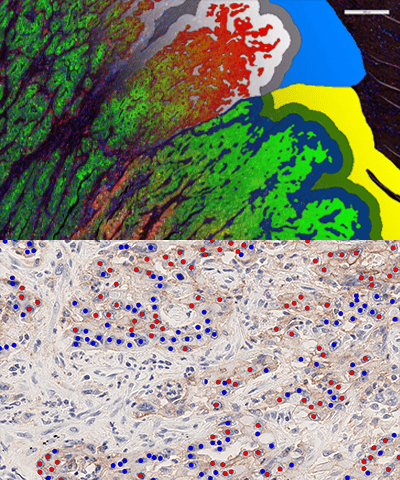
Gain the comprehensive knowledge needed for confident biomarker selection
Tailored analysis for Multiplex IF and any brightfield stains through AI based Oncotopix Discovery and Phenoplex. Have your own image analysis specialists defining the specific analysis needed to deliver the knowledge you need, with our experts ready to support yours.
Bespoke algorithm development to assist in stratifying patients for clinical trials and beyond
In case you don’t have the in house expertise to create the algorithms, our experts can develop any custom zero-click whole slide APP for you to be trial ready. Close collaboration between your experts and ours ensure optimal quality.
Seamless transition of your assay from discovery to diagnostic
Once you have a validated algorithm, the transition into the clinic should be seemless. Visiopharm provides one platform from onsite software in research to deployment of customized diagnostic algorithms.
Enable clinical trial success by
standardizing tissue-based patient stratification
Trials are often conducted multi-centric, with patient selection performed at local hospital centers. This can lead to selection inconsistencies due to variations in biomarker staining and evaluation. Standardization is crucial to ensure homogeneous patient selection and thus enable a successful trial.
Standardized patient stratification for your trial with clinical trial ready APP
Patient stratification often happens at local labs, so your algorithm needs to cover every-day-histology variation to enable a homogeneous patient selection. We ensure a validated and documented analysis for your biomarker to standardize your patient selection.
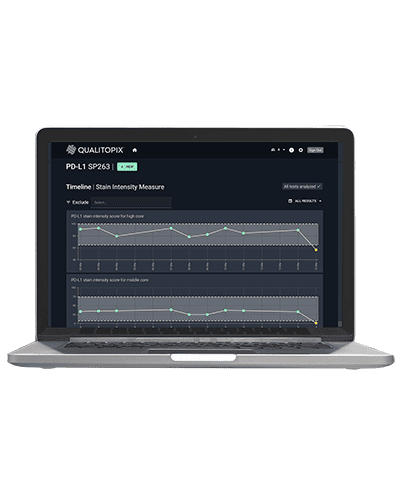
Monitor lab processes for multi-center studies
Local histology evaluation is based on local staining, which has been shown to potentially differ significantly. Qualitopix can monitor staining consistency across labs to enable standardized evaluation.
Ensuring clinical trial readiness and ongoing support for APP deployment
Our experts deliver on-site installation and pathologist training as well as ready-to-submit APP documentation to ensure a successful trial rollout.
Advance your drug and tissue assay into diagnostics
with our regulatory and deployment expertise
After successfully completing the trial, regulatory approval is needed to bring the companion diagnostics for the promising treatment to market. Proper documentation is essential to obtain certification for AI analysis. Once certified, widespread implementation across hospital labs necessitates extensive technical support and deployment experience.

Ready for regulatory demands in EU or USA
Visiopharm has an established regulatory pathway through +10 years of experience in the CE-IVD market. To this date we are the only company with a full portfolio of IVDR certified APPs.
Standardized patient selection through stain monitoring and consistent biomarker assessment
Qualitopix monitors staining consistency in any lab and the fully automated APP can be integrated in many PACS/IMS systems with full documentation and certification trail. This ensures standardized evaluation for optimal patient selection.
World-wide commercial and deployment setup ready to ensure standardized pathology interpretation
With our various representatives on multiple continents, our support is available everywhere in the world to establish global roll out strategy.

Learn how our customer Proteocyte used Visiopharm to develop a prognostic LDT test to determine, which oral precancer lesions could turn into cancer.




