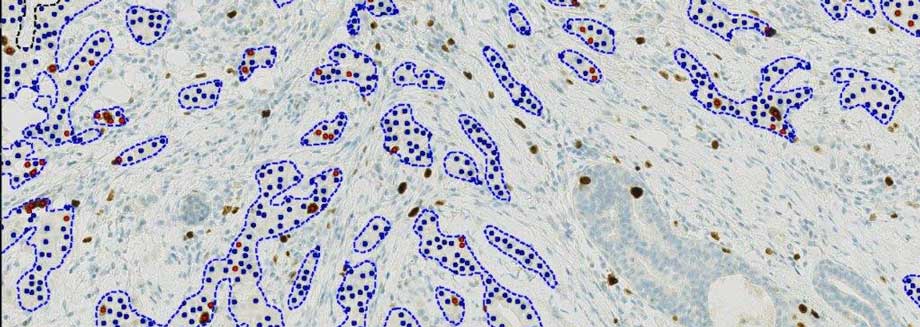
Visiopharm launches IVDR-cleared and fully automated next-generation Ki67 algorithm for IHC biomarker analysis
Visiopharm, a leading provider of AI-driven precision pathology software, has announced the launch of a next-generation Ki67 algorithm providing diagnostic decision support for pathologists. The algorithm, which has been cleared under the European In-Vitro Diagnostic Regulations (IVDR), is designed to be completely automated, allowing pathologists to become fully productive without manual steps.
Ki67 is a widely used biomarker that identifies the proliferation rate of cancer cells to determine the aggressiveness of a tumor and subsequently guide treatment decisions. To support optimal treatment decisions, standardization is required to minimize the impact of inter- and intra-reader variability. More recently, the Ki67 protein expression has also been shown to correlate with response to immune checkpoint inhibitors, thus potentially granting Ki67 an important role in the deployment of precision medicine.
“The Visiopharm algorithm is helping us achieve standardization in the scoring of Ki67 for breast cancer patients. We look forward to having the algorithm fully automated and integrated into our workflow saving us time and effort while also improving accuracy and consistency in our results”, said Paul van Diest, Head of Pathology at UMC Utrecht.
Agilent Technologies Inc. had approached Visiopharm to validate assays for the Omnis platform, and the next generation Ki67 algorithm has now been added to the growing portfolio of fully automated workflow APPs validated for and operable with Agilent’s systems.
Lou Welebob, Vice President and General Manager for Agilent’s Pathology Division, Diagnostic and Genomics Group, stated, “We decided to develop AI-based APPs for Her2, Ki67, and PD-L1 for our assays and the Omnis platform. HER2 was the first with IVDR clearance followed by Ki67, and PD-L1 is not far behind. We believe this level of automation is required to deploy AI-driven precision pathology at scale, and Visiopharm has a go-to-market strategy that will enable accelerated deployment of computational pathology. The fact that Visiopharm already has several IVDR-cleared digital assays on the market and is fully committed to obtaining FDA clearance as well, made this collaboration ideal for Agilent.”
Automation of the Ki67 algorithm removes key barriers for adoption in routine use by incorporating multiple steps: detection of patient tissue, elimination of slide artifacts (tears, folds, pen-marks, out-of-focus, etc.), robust identification of invasive tumor cells, cell classification, quantification, and visualization of tumor heterogeneity (heatmaps and hot-spots).
“We are thrilled to launch this next-generation Ki67 algorithm. We believe that automation of the full image analysis pipeline will have a significant impact on the adoption of AI in routine use. We are committed to making this technology widely available to pathologists, eliminating the adoption and activation barriers that has so far been a challenge to achieve immediate productivity and a measurable return on investment”, said Dirk Vossen, Chief Diagnostics Officer at Visiopharm.
About Visiopharm
Visiopharm® is a leading provider of AI-driven precision pathology software for research and diagnostics. In research, it is a technology leader providing tools that help scientists, pathologists, and image analysis experts produce accurate data for all types of tissue-based research. In diagnostics, it is a world leader within clinical applications, with no less than eight diagnostic algorithms cleared under IVDR for EU customers. These applications provide diagnostic decision support and quality assurance and can be easily activated and integrated into existing lab workflows.
Founded in 2002, Visiopharm is privately owned and operates internationally with over 750 customer accounts in more than 40 countries. The company’s headquarters are located in Denmark’s Medicon Valley, with offices in Sweden, the UK, Germany, the Netherlands, and the United States, and local representation in France and China.
For enquiries:
Johanne Louise Brændgaard
Chief Marketing Officer
jlb@visiopharm.com

