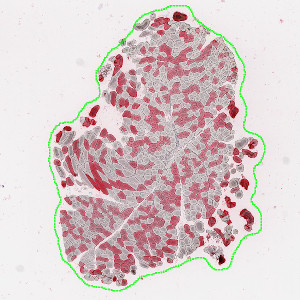
Tissue section delineated with the Tissue Detection part of the APP.
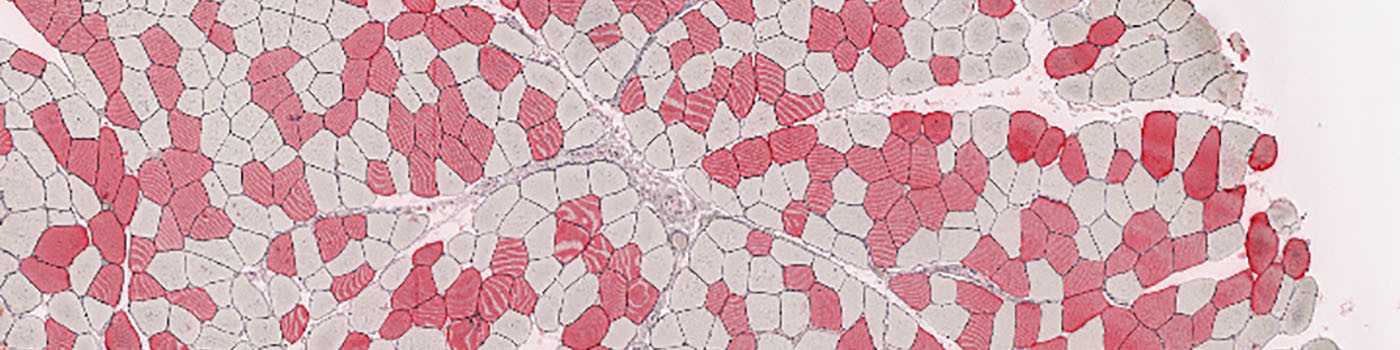
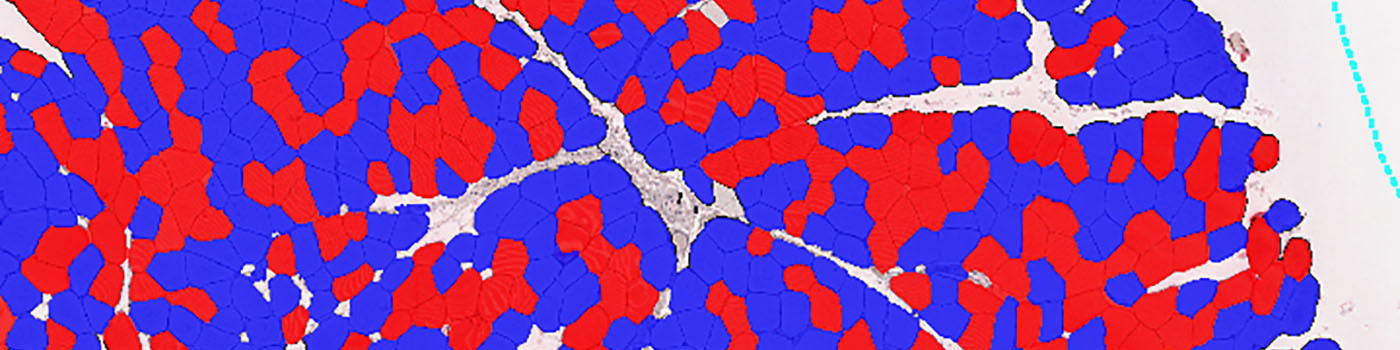
#10078
Skeletal Muscle atrophy is a hallmark change associated with muscle degeneration, disuse atrophy and neurogenic atrophy. Fiber type grouping is a hallmark pathologic change associated with motor neuron atrophy and synaptic loss, see [1].
These APPs identify individual muscle fibers in whole slide images and calculate the lesser diameter for each fiber. This is done for both stained and unstained fibers allowing quantification of muscle fiber diameter by myosin fiber type. In type I myosin predominant muscle, the clustering of type II fibers can be assessed as a measure of synaptic denervation and reinnervation.
Auxiliary APPs
Auxiliary APPs are used for additional process steps, e.g. finding Region of Interest (ROI).
APP: 01 – Tissue Detect
This auxiliary APP is used to automatically detect tissue sections on a virtual slide. It will select the N largest tissue sections and delineate them with a region, which can be used for the quantification step.
Quantitative Output variables
From the delineated muscle fiber profiles and clusters, the following output variables are obtained from this protocol:
Workflow
Step 1: Load the auxiliary APP for tissue detection “01 – Tissue Detect’
Step 2: Load the analysis protocol “02 – Analyze’
Step 3: Click the save button to transfer the results to the database.
Methods
This APP is designed for usage on virtual slides with multiple tissue sections on each slide. The APP includes a tissue detection step and a quantification step. The tissue detection step can be configured to locate the N largest tissue sections for quantification.
In the Quantification step each individual muscle fiber profile is delineated by using fiber staining, combined with detection of inner fiber texture and elongated fiber border structures. After fiber delineation, clusters of positive stained fibers are located. Clusters are defined as groups of 4 or more fibers in contact with each other.
Staining Protocol
Muscle samples of tibialis and/or soleus muscle were formalin fixed and paraffin embedded prior to being sectioned at a 5μm thickness.
To outline individual muscle fibers with reticulin, slides were stained using the Ventana Reticulum kit protocol #11, where the slides are deparaffinized, incubated with chromagen for 16 minutes, and followed by a series of washes. Post histochemical staining, slides are rinsed in 95% alcohol to make sure the liquid coverslip (oil) has been completely removed, and wet-loaded onto the Leica Bond RX autostainer for immunohistochemistry.
Epitope retrieval was performed using pH6 citrate buffer for 10 minutes. Background is blocked using Rodent Block M (Biocare cat#RBM96LL) for 30 minutes, followed by a 20 minute primary antibody incubation (Fast Myosin Novus Biologicals cat#NBP1-22811, MY32 dilution 1:3 in Bond diluent or Slow Myosin Millipore cat#MAB1628, dilution 1:400 in Bond diluent). Then a Mouse on Mouse AP polymer (Biocare MM624H) was applied for 20 minutes, detected by Refine Red chromagen as per standard Bond protocol (Bond Polymer Refine Red Detection kit, protocol MoM AP).
Keywords
Skeletal Muscle, Muscle Atrophy, Neurogenic Atrophy, Muscular Dystrophy, Dystrophic Fibers, Tibialis, Soleus
References
USERS
This APP was developed for, and validated by, Pavan K. Auluck MD, PhD and Robert W. Dunstan, DVM, MS, DACVP Translational Pathology Laboratory, Biogen Idec, Cambridge, MA, USA.
LITERATURE
1. Dubowitz, V., et. al., (2013). Muscle Biopsy. A Practical Approach (4th Edition) Udgivelsessted: Sainders Ltd.